Topic 6 - radioactivity
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
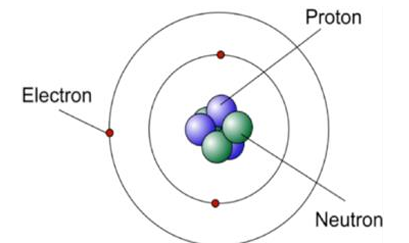
atom
a positively charge
made of positive protons
and neutral neutrons
surrounded by negatively charged electrons
the electrons orbit the nucleus at different fixed distances from the nucleus
the nuclear radius is a lot smaller than the radius of the atom
almost all the mass of the atoms lies in the nucleus
subatomic particle, relative mass, relative charge
proton, 1, +1
neutron, 1, 0
electron, 0.0005, -1
positron, 0.0005, +1
size of an atom
~0.1 nanometres, 10-10
isotopes and elements
atoms of the same element have the same number of protons
neutral atoms have the same number of electrons and protons
isotopes are atoms of the same element, but with different masses
they have the same number of protons but different number of neutrons
for example, Carbon-12, Carbon-13 and Carbon-14
X is the letter of their element
A is the mass number (number of neutrons and protons)
Z is the proton number
N is the charge
on a neutral atom, electrons = protons, so cancels out
if there are N more electrons than protons, then the charge is -N
if there are N fewer electrons than protons, then the charge is +N
the number of protons does not change for a certain element
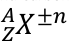
atoms and EM radiation
when electrons change orbit (move closer or further away from the nucleus)
when electrons move to a higher orbit (further from the nucleus)
the atom has absorbed EM radiation
when the electrons fall to a lower orbit (closer to the nucleus)
the atom has emitted EM radiation
if an electron gains enough energy, it can leave the atom to form an ion
radiation/forms of decay
decay occurs in a random process
forms of decay:
alpha (a helium nucleus)
highly ionising
weakly penetrating
beta minus (electron)
medium ionising
medium penetration
beta plus (positron)
medium ionising
medium penetration
gamma (radiation)
low ionising
highly penetrative
neutrons
background radiation
weak radiation that can be detected from natural/external sources
examples of background radiation include:
cosmic rays
radiation from underground rocks
nuclear fallout
medical rays
methods of measuring radioactivity
photographic film
film goes darker when it absorbs radiation - the more radiation absorbed, the darker it gets (the film is initially white)
worn as badges by people who work with radiation, to check how much exposure they have had
Geiger-Muller tube
a tube which can detect radiation
each time it absorbs radiation, it transmits an electrical pulse to the machine, which produces a clicking sound
the greater the number of clicks per second (frequency of clicks) the more radiation is present
atom structure - Dalton and JJ Thomson
1800 - Dalton said everything was made of atoms
1897 - JJ Thomson discovered the electron
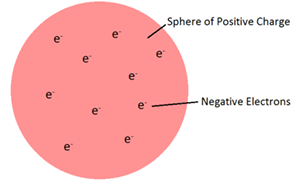
the Plum Pudding Model was formed
the overall charge of an atom is neutral, so the negative electrodes were dispersed through the positive “pudding” to cancel out the charges
atomic structure - Rutherford
1911 - Rutherford realised most of the atom was empty space
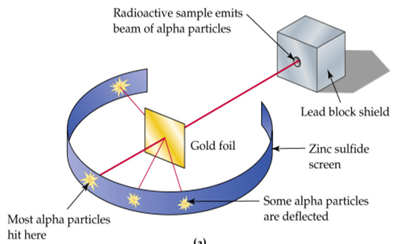
The Gold Foil experiment
this experiment was carried out by Geiger and Marsden
most particles went straight through
so most of atom is empty space
some α particles were slightly deflected
so nucleus must be positive, repelling positive α
few α particles were deflected by >90°
so nucleus contained most of the mass
atomic structure - Rutherford model
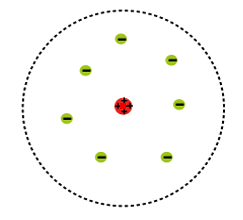
1913 - Rutherford model
now there is a positive nucleus at the centre of the atom, and negative electrons existing in a cloud around the nucleus
atomic structure - Bohr
1913 - Bohr produced the final model of the atom
if Rutherford was right, the electrons in the cloud close to the nucleus would get attracted and cause the atom to collapse. therefore, he concluded that the electrons exist in fixed ‘orbitals’
decay processes
Beta-Minus Decay
neutron becomes a proton, and releases an electron
Beta-Plus Decay
proton becomes a neutron, and releases a positron
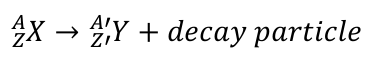
alpha
an alpha particle is equivalent to a helium nucleus
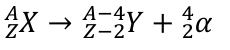
beta
a beta particle is an electron emitted from the nucleus
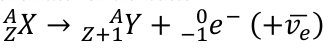
gamma
a gamma ray is electromagnetic radiation
nuclei after decay often have excess energy, which they release as gamma when the atom undergoes nuclear arrangement
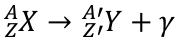
activity
activity is the number of decays in a sample per second
activity is initially very high (the more atoms in the sample, the greater the chance of at least one of them will decay)
activity decreases exponentially over time
units of activity are Becquerel, Bq
half life
the half life of an isotope is the time taken for half the nuclei in a sample to decay
or the time taken for the activity of a sample to decay by half
it cannot be predicted when any one nucleus will decay, but the half life is a constant that enables the activity of a very large number of nuclei to be predicted during the decay
net decline
work out ratio of net decline of radioactive nuclei after X half lives
half the initial number of nuclei, and keep doing so X number of times
net decline = (initial number - number after X half lives)/initial number
example: there were initially 80 nuclei, with a half-life of 15 minutes, net decline after 3 half-lives?
80 → 40 → 20 → 10
1st life, 2nd, 3rd half life
so net decline = (80-10)/80 = 7/8
uses of radioactivity
smoke alarms
Americium is used in smoke alarms
Americium has a half life of 432 years
it is an alpha emitter
this is stopped by a few centimetres of air (as it is weakly penetrating)
the alpha particles ionise air particles and makes them charged therefore making a current
if smoke enters the air around the alarm, the current drops in the circuit
causing the alarm to sound
irradiating food
gamma rays transfer energy to bacteria, killing them and sterilising food
also used to delay ripening of fruit
sterilisation of equipment
radiation, usually gamma, exposed onto equipment to kill all microbes present on the equipment, so they are safe for operations
tracing and gauging thickness
beta radiation is mildly penetrating and can just pass through paper
a source and receiver are placed either side of the paper during its production
if there is a drop or rise in recieved electrons, then that means the thickness of the paper has changed - ie. a defect in the production
it is also used inside pipes, with a detector placed externally, to measure the thickness of walls of the pipe
diagnosis and treatment of cancer
consuming/injecting a gamma emitter, it passes through the body and an external detector can picture where the tracer has collected in the body, which can reveal tumours
gamma rays are used on the tumour, killing the cancer cells
however, exposing rays on healthy cells can cause them to possibly mutate or cause damage
dangers of ionising radiation
a short half life
the source presents less of a risk, as it does not remain strongly radioactive
this means initially it is very radioactive, but quickly dies down
so presents less of a long term risk
long half life
the source remains weakly radioactive for a long period of time
Americium is suitable in smoke alarms because it will not need to be replenished, and its weak activity means it won’t be harmful to anyone
its half life is 432 years
safety measures
limiting patient does
only use radioactive tracers with a short enough half life
so short enough to quickly be removed over a day or so
but long enough to still be detectable after the time taken for it to pass through the body
common medical tracers used have a half life of 6 hours
limiting risks to medical personnel
they leave the room during radioactive tests, as their everyday close proximity to the radioactive sources puts their health at risk in the long term
difference in radiation
contamination
lasts for a long period of time
the source of the radiation is transferred to an object
eg. radioactive dust settling on your skin (your skin becomes contaminated)
irradiation
lasts only for a short period of time
the source emits radiation, which reaches the object
eg. radioactive dust emitting beta radiation, which “irradiates” your skin
medical items are irradiated sometimes to kill bacteria on its surface, but not to make the medical tools themselves radioactive
treatment of tumours
external
a beam of gamma radiation (usually a wide beam) rotates around the body
it continually focuses on the tumour, while only passing momentarily across healthy cells surrounding the tumour
this ensures minimal damage occurs on the healthy cells, while the tumour is most affected
however it takes a long time to fully treat the tumour, taking multiple visits for around 5 weeks
there is a greater risk of long term side effects, as the radiation passes through healthy tissues
internal
radioactive material is held within a needle, and is injected directly into the tumour
a longer period of time needs to be spend in hospital, as some radioactive implants are of high radioactivity (so you emit radiation, requiring you to have very limited contact with visitors until the source’s activity has decreased)
PET scanners
positron emission tomography
radioactive tracer is inserted into the body
the tracer is tagged to the desired chemical, and the tracer therefore travels in the body where this certain chemical travels (eg glucose or ammonia)
the scanner records where the tracer emits radiation
this produces a live 3D visualisation of the body
used to show how effective current treatment is
or to diagnose cancer, epilepsy, Alzheimer’s
isotopes
the isotope used in PET scanners is made locally just before insertion
this is because the tracer has a half life of 110 mins, so it cannot be stored for long before it decays
nuclear power
uranium fuel splits releasing neutrons, which are absorbed by further uranium nuclei, which split (this is fission, in a chain reaction)
no carbon dioxide is produced
there is a safety risk of radiation leaking, or the chain reaction become uncontrollable and causing a meltdown
also, a security risks as terrorists can try and obtain the radioactive material
public perception of nuclear power is negative, due to the fatal disasters caused by nuclear power plants
waste disposal is difficult - initially extremely hot, the waste needs to be placed deep in lakes, ‘cooling ponds’ to cool down, before being stored deep underground (it can be used for nuclear warheads so is a terrorist risk) for centuries
nuclear energy
fusion is the process of small nuclei being forced together (under immense temperature and pressure) to form a heavier nucleus
this is the energy source for stars
the electrostatic repulsion for the protons in the two different nuclei means a lot of energy is required to brings the nuclei close enough to fuse
so fusion cannot happen at low temperatures and pressures
so this makes it very difficult to make a practical and economic fusion power station
fission is the process of a nucleus splitting into two smaller nuclei after absorbing neutrons, which releases more neutrons
Radioactive Decay is when an unstable nucleus decays into two smaller nuclei
all these processes release energy, and can be a source of energy
U-235 fission
Uranium-235 (this means 235 nucleons) is the fuel used in nuclear (fission) power stations
it absorbs neutrons, and becomes unstable
this causes it to undergo fission
forming two ‘daughter’ nuclei
the products are radioactive, as they are strong gamma emitters (some of the energy released from the fission is also held by the daughter nuclei)
emitting two or more neutrons as well
chain reaction
after one nucleus splits, emitting neutrons, these neutrons cause further fissions, which releases more neutrons…this continuous process is a chain reaction
this needs to be controlled
as more neutrons are released than the number absorbed, this could cause an exponential process
for example the first fission releases two neutrons, which will cause 2 fissions, releasing four neutrons, causing four fissions, releasing eight neutrons…and so on, which would cause a meltdown
moderators
this is usually water/graphite, and slows down the emitted neutrons to be absorbed for further fissions (fast moving neutrons cannot be easily absorbed)
control rods
these are boron rods in the reactor core, which absorb excess neutrons, preventing a runaway chain reaction
the heat energy from the chain reaction is absorbed by water (coolant) which evaporated into steam, and is used to turn a turbine, which turns the generator which generates electricity