3.2. HYDROCARBONS
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Why are arenes highly stable, and why do they undergo electrophilic substitution instead of addition reactions?
Stability of Arenes:
Arenes, such as benzene, have delocalized π-electrons spread over the ring, giving extra stability.
The aromatic system remains intact during reactions, preventing disruption of this stabilisation.
Electrophilic Substitution:
Electrophiles attack the benzene ring, replacing hydrogen atoms without disturbing delocalisation.
Unlike alkenes, arenes do not undergo addition reactions because these would break aromatic stability.
How do arenes undergo halogenation, and how does the presence of alkyl groups affect substitution positions?
Halogenation Reaction:
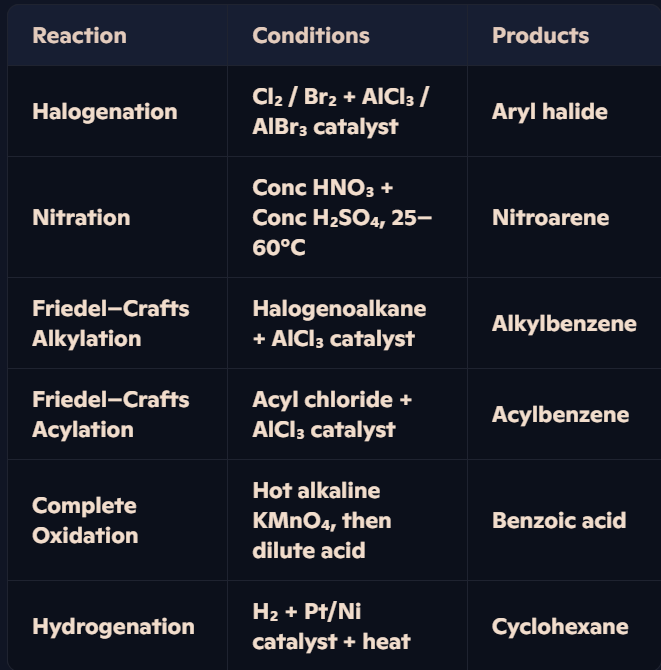
Arenes react with Cl₂ or Br₂ in the presence of AlCl₃ or AlBr₃ catalysts to form halogenoarenes (aryl halides).
The catalyst polarises the halogen, making it a stronger electrophile that substitutes hydrogen on benzene.
Effect of Alkyl Groups (Methylbenzene Example):
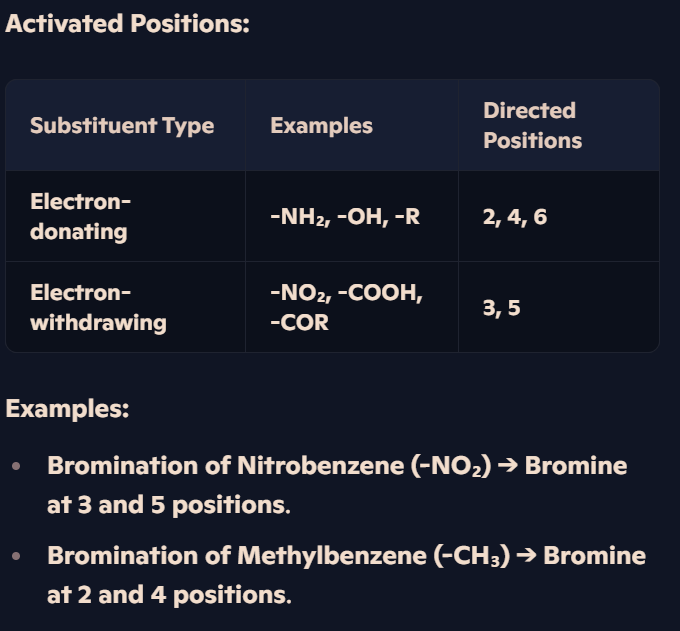
Electron-donating alkyl groups (like -CH₃ in methylbenzene) activate the 2 and 4 positions, leading to directed substitution.
Excess halogen results in multiple substitutions at these positions.
How does the nitration of benzene and methylbenzene occur, and what is the effect of alkyl groups?
Nitration Reaction:
Benzene reacts with a mixture of concentrated HNO₃ and H₂SO₄ at 25–60°C.
Sulfuric acid acts as a catalyst, generating the NO₂⁺ electrophile, which substitutes a hydrogen atom on benzene.
Effect of Alkyl Groups (Methylbenzene Example):
Alkyl groups activate the 2 and 4 positions, leading to directed nitration at these locations.
What are Friedel–Crafts reactions, and how do alkylation and acylation modify benzene’s reactivity?
Friedel–Crafts Reactions:
Used to introduce alkyl (-R) or acyl (-CO-R) groups into benzene via electrophilic substitution.
Alkylation:
Reagents: CH₃Cl + AlCl₃ catalyst + heat.
Mechanism: Alkyl cation attacks benzene, replacing hydrogen with an alkyl group.
Example: Benzene reacts with chloropropane (CH₃CH₂CH₂Cl) to form propylbenzene.
Acylation:
Reagents: CH₃COCl + AlCl₃ catalyst + heat.
Mechanism: Acyl cation attacks benzene, replacing hydrogen with an acyl group.
Example: Methylbenzene reacts with propanoyl chloride to form an acyl benzene, with substitution at the 4-position due to the methyl (-CH₃) group.
How are alkyl side chains in arenes oxidised, and what are the final products?
Oxidation Reaction:
Hot alkaline KMnO₄ followed by dilute acid oxidises alkyl side-chains to carboxylic acids.
Benzene ring remains intact, and the side-chain is converted to a benzoic acid.
Example:
Ethylbenzene → Benzoic acid (after oxidation with KMnO₄ and H₂SO₄).
How does the hydrogenation of benzene and methylbenzene occur, and what effect does it have on aromaticity?
Hydrogenation Reaction:
Reagents: H₂ + Pt/Ni catalyst + heat.
Mechanism: Benzene loses its aromatic stability, converting to cyclohexane.
Effect on Methylbenzene:
Methylbenzene undergoes hydrogenation to form cycloethylbenzene.
Loss of aromaticity, but alkyl groups remain attached.
What are the key reactions of arenes, their conditions, and products?
What are the key steps in the electrophilic substitution mechanism of arenes, as exemplified by the formation of nitrobenzene and bromobenzene?
Step 1: Generation of the Electrophile
Arenes have a stable delocalised π-system, meaning electrophiles must be highly reactive.
Electrophiles used:
Halogenation: X⁺ (e.g., Br⁺)
Nitration: NO₂⁺
How Electrophiles Are Formed:
Halogenation: Br₂ reacts with AlBr₃ → Br⁺ electrophile.
Nitration: Conc. HNO₃ + Conc. H₂SO₄ → NO₂⁺ electrophile.
Step 2: Electrophilic Attack
Benzene donates a pair of electrons to form a covalent bond with the electrophile.
This disrupts aromaticity, leaving only four π-electrons and a positively charged intermediate.
Step 3: Restoring Aromaticity
Heterolytic cleavage of the C–H bond occurs.
Bonding electrons re-enter the benzene π-system, restoring aromaticity.
Example Reactions:
Bromination: Benzene + Br₂ + AlBr₃ → Bromobenzene.
Nitration: Benzene + HNO₃ + H₂SO₄ (reflux, 25–60°C) → Nitrobenzene.
How does delocalisation (aromatic stabilisation) explain why arenes undergo substitution instead of addition reactions?
Aromatic Stability:
Benzene has a delocalised π-system with extra stability.
Addition reactions break this delocalisation, leading to energy loss.
Electrophilic Substitution vs. Addition:
Substitution: Retains aromaticity (electrons restored via heterolytic cleavage).
Addition: Destroys aromaticity (π-electron system disrupted).
Example: Hydrogenation of Benzene
H₂ + Pt/Ni catalyst + heat → Cyclohexane (loses aromaticity, less stable).
How do reaction conditions determine whether halogenation occurs in the aromatic ring or the side-chain of arenes?
Halogenation in the Aromatic Ring:
Occurs when a halogen (Cl₂ or Br₂) is used with a halogen carrier catalyst (AlCl₃ or AlBr₃).
Involves electrophilic substitution, generating a halogen electrophile (e.g., Br⁺).
Example: Bromination of methylbenzene → Bromomethylbenzene.
Halogenation in the Side-Chain:
Occurs when the halogen is passed into boiling alkylarene in the presence of UV light.
Involves free-radical substitution, forming halogenated side-chains.
Example: Methylbenzene + Cl₂ (UV light) → Chloromethylbenzene.
Effect of Excess Halogen:
In the side-chain: Every hydrogen is replaced with a halogen atom.
In the ring: Multiple substitutions occur at 2 and 4 positions (if an activating group is present).
How do different substituents affect the position of electrophilic substitution in arenes?
Substituents Influence Reactivity:
Electron-donating groups (-NH₂, -OH, -R): Increase electron density, making the ring more reactive.
Electron-withdrawing groups (-NO₂, -COOH, -COR): Reduce electron density, making the ring less reactive.