Gas Laws
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
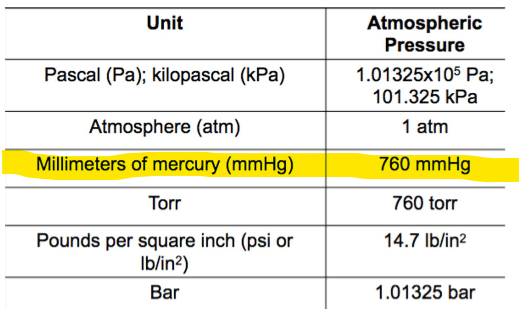
Atmospheric pressure at sea level in Pa, kPa, atm, mmHg, Torr, Pounds per square inch, Bar
What percentage of air is made up of what components
Nitrogen = 78%
Oxygen = 21%
CO2 = 0.033%
Water vapour, krypton, argon, … make up the rest
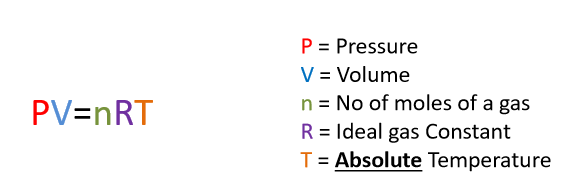
Universal Gas Law formula & units
P = Pa
V = m3
n = No. of moles
R = 8.31 (ideal gas constant)
T = Kelvin (°C + 273)
Boyle’s Law words
At a constant temperature, the pressure exerted by a constant number of gas molecules is inversely proportional to volume of the container
(Boyle is constantly boiling hot!)
Boyle’s Law formula
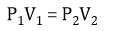
Volume and the number of moles of a gas are ______ proportional
directly
Volume and temperature of a gas are ______ proportional
directly
Volume and the pressure of a gas are ______ proportional
inversely
P ∝ 1/V
Charles’ Law words
The volume of a gas at a constant pressure varies directly with absolute temperature at a constant pressure
Charles’ Law formula
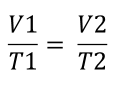
What is BTPS correction & what does it do
BTPS = conditions within the lungs (body temperature, pressure, water vapor saturation)
Converts flow and volume measured at ambient conditions to the conditions within the lungs
(BTPS = Body Temperature and Pressure, Saturated)
Ambient conditions
Called ATP (ambient temp & pressure)
Ideal gas law formula
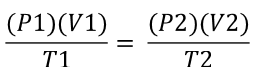
Dalton’s Law
The total pressure of a mixture of gases is the sum of the partial pressures of each gas
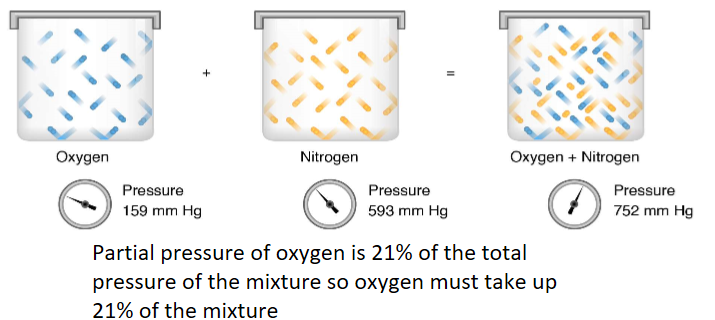
Is the % of oxygen compared to other components greater, lesser or the same as at sea level compared to the top of Everest
There is still the same % of oxygen at the top of Everest, but partial pressure is reduced so total oxygen volume is reduced
Water vapour percent in inspired, alveolar & expired air
Inspired: 0.5%
Alveolar: 6.2%
Expired: 6.2%
Henry’s Law
When a gas under pressure comes in contact with a liquid, it dissolves in the liquid until equilibrium is reached
(the greater the partial pressure, the greater the amount that will dissolve)
Rank these in order of solubility: CO2, O2, N2
