Unit 5: Moles and Stoich
1/17
Earn XP
Description and Tags
im off track and theres a math test in two days WHAT DO I DO HELLPP. its 5/11,... I hate moles and stoich. I hate chem in general
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Why do we use moles?
Atoms cant be measured. Moles is the unit for amount
Avogadro's Constant
6.02 E23, in Section 2 molescules are in a mol
Mole calc:
grams/molar mass
Or
Mols of gas: volume/molar volume (constant at STP)
How to calculate Emperical formula
Calculate to mols, divide by smalles mols.
How to find molecular formula
Find emperical formula, take mass of moleuclar/emperical. mult this number by everything
Percent Composition of Percent by mass
part/whole times 100
% composition of water in a hydrate
(Mass of water/mass of hydrate) times 100
What is an anhydrate (its anhydrous)
water is removed
How to know all water has been taken out of hydrate?
Heat to a constant mass
Percent Yield
experimental/theoretical x 100
Why do we care about % yield?
Low yield means more waste
Green chemistry: we need high yields
Avogadro’s Law
Equal volumes of all gasses, when measured at the same temperature and pressure, contain an equal number of particles (moles)
Combined Gas Law
P1V1/T1 is equal to the final, P2V2/T2
Temperature must be in Kelvin
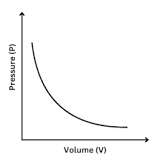
Boyles Law
At constant temperature, the volume of a gas is inversely related to the pressure
Charles Law and Gay-Lussac’s Law
Direct relationship, they each get rid of one thing off the combined gas laws.
Ideal Gas Equation!
PV=nRT
Pressure in Pa. Volume in cm3
n is moles. r is constant. Temp in Kelvin
crazy thing about Pvnrt
Pressure is in pa, so you x1000 if u need it in kpa
Volume is in cm3 so you /1000 if u need dm3
Temp NEEDs to be in Kelvin
Ideal gas MODEL
Gas particles have no intermolecular forces of attraction
volume of gas particles is negligible (lot of space in between)
Gasses have free moving particles