Chem Molecules
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
Cations
Ion with positive charge (formed when an atom loses one or more electrons)
Anions
Ion with a negative charge (formed when an atom gains an electron)
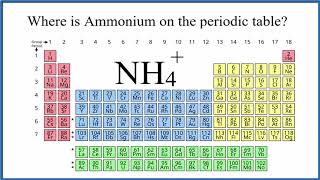
NH4+
Ammonium
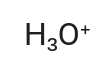
H3O+
Hydronium
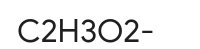
CH3COO- or C2H3O2-
Acetate
CN-
Cyanide
OH-
Hydroxide
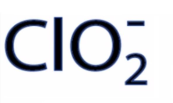
ClO2-
Chlorite

ClO3-
Chlorate

ClO4-
Perchlorate
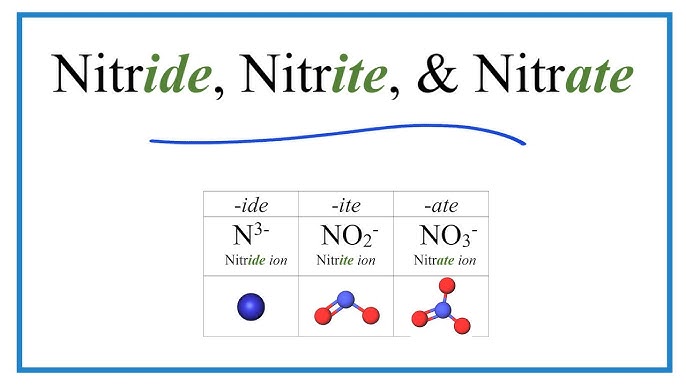
NO2-
Nitrite
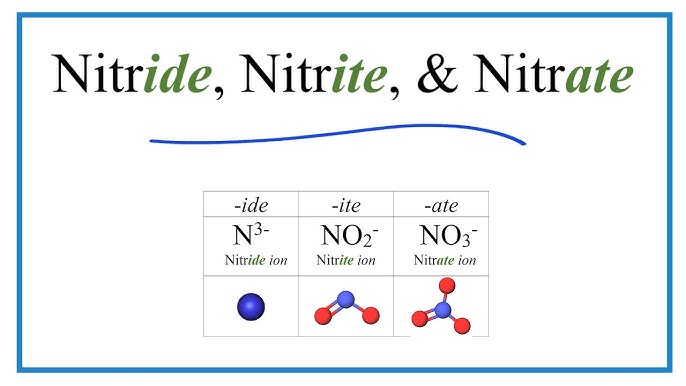
NO3-
Nitrate
MnO4-
Permanganate
CO₃²⁻
Carbonate
HCO3-
Hydrogen carbonate or bicarbonate
CrO42-
Chromate
Cr2O72-
Dichromate
O22−
Peroxide ion
PO₄³⁻
Phosphate
[H2PO4]−
Dihydrogen phosphate
SO₃²⁻
Sulfite
SO₄²-
Sulfate
HSO4-
Hydrogen sulfate (bisulfate)
H 2O 2
Hydrogen peroxide
NH4
Ammonia
N 2H 4
Hydrazine
Nitric oxide
NO
Nitrogen dioxide
NO2
SO2
Sulfur dioxide
Sulfur trioxide
SO3
Carbon monoxide
CO
Carbon dioxide
CO2
Chlorine monoxide
ClO
Disulfur decaflouride
S2F10
HCl
Hydrochloric acid
HBr
Hydrobromic acid
H₂S
Hydrogen sulfide
H 3PO 4
Phosphoric acid
HNO₃
Nitric acid
HNO2
Nitrous acid
H₂SO₄
Sulfuric acid
H₂SO₃
Sulfurous acid
HClO₄
Perchloric acid
HClO₃
Chloric acid
HClO2
Chlorous acid
HClO
Hypochlorous acid
HBrO 4
Perbromic acid
HBrO3
Bromic acid
HBrO2
Bromous acid
HBrO
Hypobromous acid
two forms: orthoperiodic acid (H5IO6) and metaperiodic acid (HIO4)
Periodic acid
HIO3
Iodic acid
HIO2
Iodous acid
HIO
Hypoiodous acid
CH₄
Methane
C₂H₆
Ethane
C₃H₈
Propane
C₄H₁₀
Butane
C₅H₁₂
Pentane
C₆H₁₄
Hexane
C₇H₁₆
Heptane
C₈H₁₈
Octane
C₉H₂₀
Nonane
C₁₀H₂₂
Decane
[SCN] −
Thiocyanate
AsO₄³⁻
Arsenate
C2O4(2−)
Oxalate
IO-1
Hypoiodite
CO₃²⁻
Carbonate
C8H4O4-2
Phthalate
CN-
Cyanide
HO4S-
Bisulfate
HO3S-
Bisulfite
Cr2O7-2
Dichromate
HSO4-
Hydrogen sulfate
CH2O
Formaldehyde
C2H4O2
Acetic acid
C3H6O3
Lactic acid
C4H8O4
Erythrose
C5H10O5
Ribose
C6H12O6
Glucose
Acetylene
C2H2