Pathology Infectious Disease 3=Viruses
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
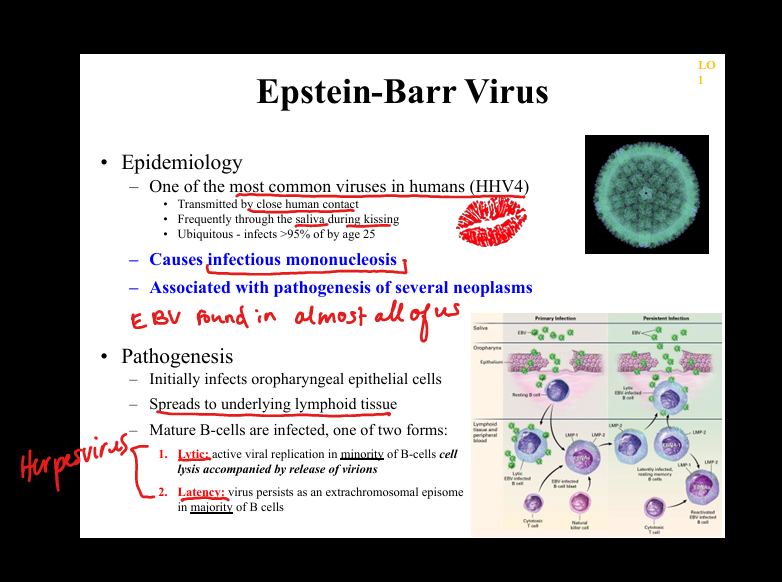
What is the natural history of primary EBV infection?
Early infection of oropharyngeal epithelium(fever, sore throat, maliase, swollen glands) → spread to lymphoid tissue → infection of B cells → lytic replication in a minority of B cells + latent infection in most B cells → strong CD8+ T‑cell response → clinical infectious mononucleosis.

What are the phases of EBV clinicopathologic manifestations?
Primary infection (mono), latency (episomal EBV in memory B cells), reactivation (immunosuppression/immunosenescence), EBV‑driven neoplasia (e.g., DLBCL, Burkitt, Hodgkin, nasopharyngeal carcinoma).
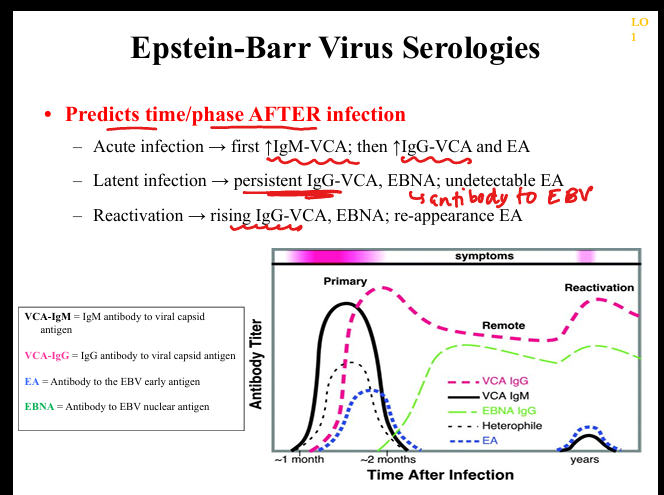
What are the key serologic markers in EBV infection?
VCA‑IgM (acute), VCA‑IgG (acute → persists for life), EA (acute/reactivation), EBNA (appears late, persists for life), heterophile antibodies (Monospot; positive in most adolescents/adults).

What immune abnormalities occur in EBV infection?
Marked CD8+ T‑cell activation (atypical lymphocytes), transient immune dysregulation, autoantibodies (e.g., anti‑platelet), risk of uncontrolled B‑cell proliferation if T‑cell response is impaired.
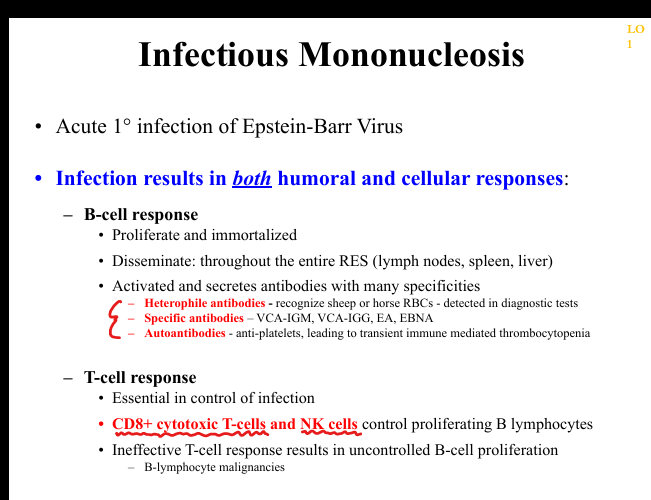

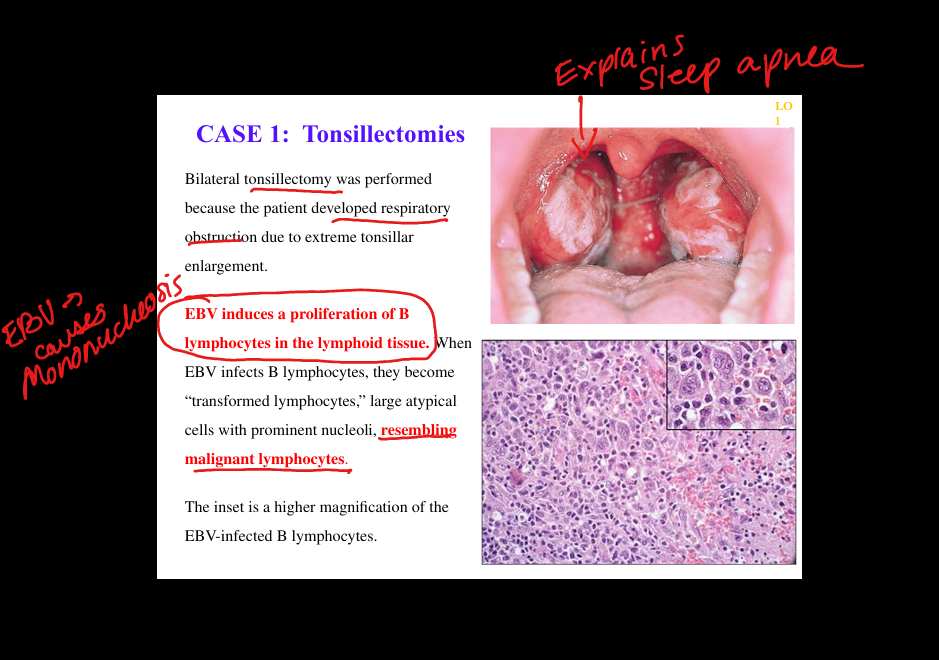
What are the histopathologic findings in EBV infectious mononucleosis?
Peripheral blood shows atypical CD8+ T lymphocytes; lymph nodes show paracortical expansion; tonsils show EBV‑infected large B‑cell blasts; spleen enlarged with white/red pulp expansion.


What is the pathogenesis of HSV‑1/HSV‑2 latency?
Primary infection in mucocutaneous epithelium → retrograde transport to sensory ganglia → lifelong latency → periodic reactivation → vesicular lesions; HSV evades immunity via downregulating MHC.
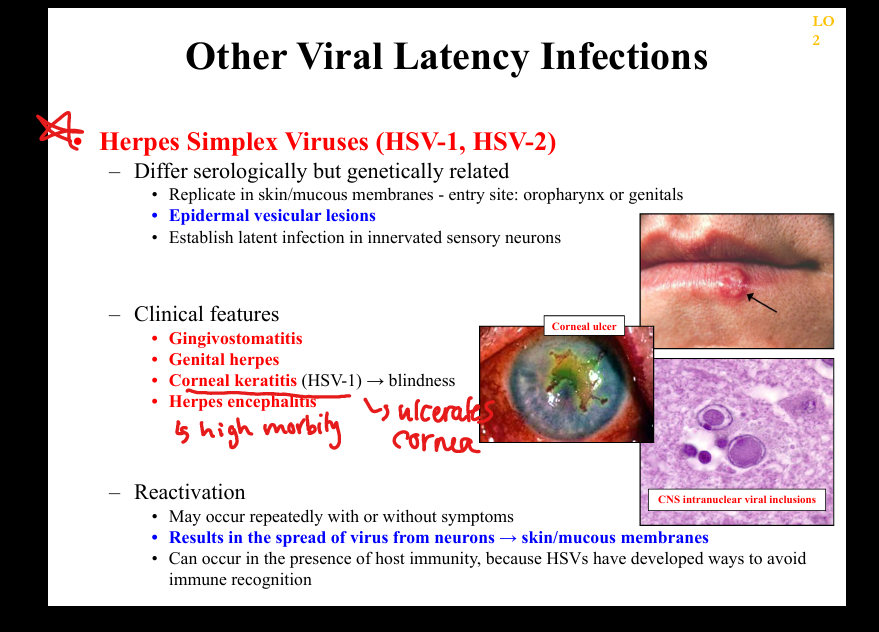

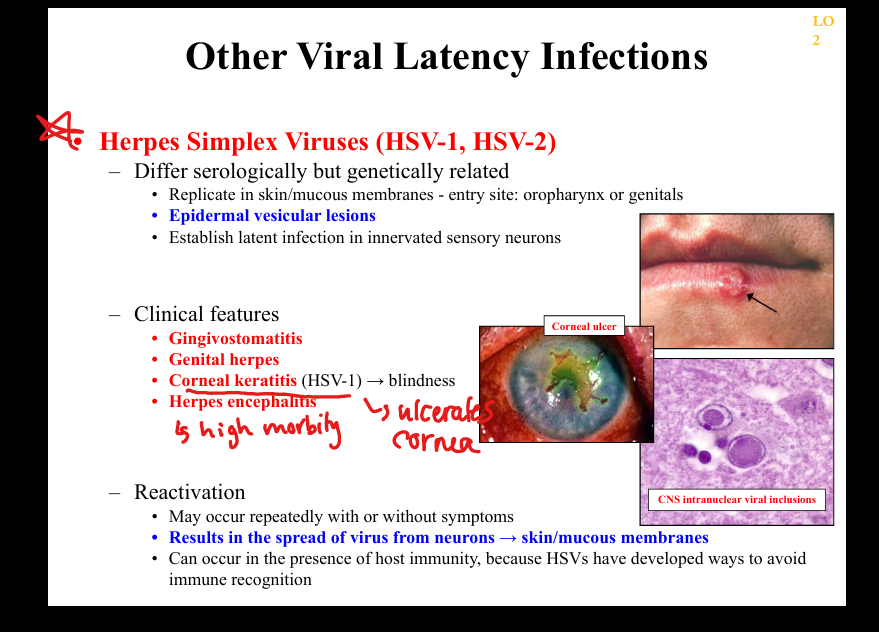
What clinicopathologic changes occur in HSV latency/reactivation?
Recurrent oral/genital vesicles, gingivomatitis, corneal keratitis (HSV‑1)=>blindness, encephalitis, painful dermatomal recurrences; histology shows multinucleated giant cells and Cowdry A inclusions.

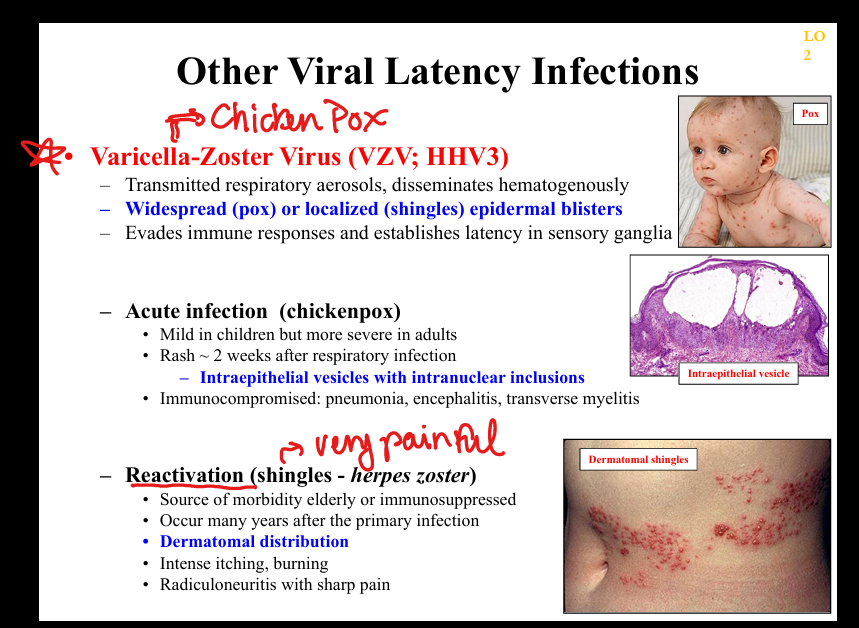
What is the pathogenesis of VZV latency?
Primary infection (chickenpox) → viremia → latency in dorsal root ganglia → reactivation as shingles; dermatomal vesicles with severe neuropathic pain.
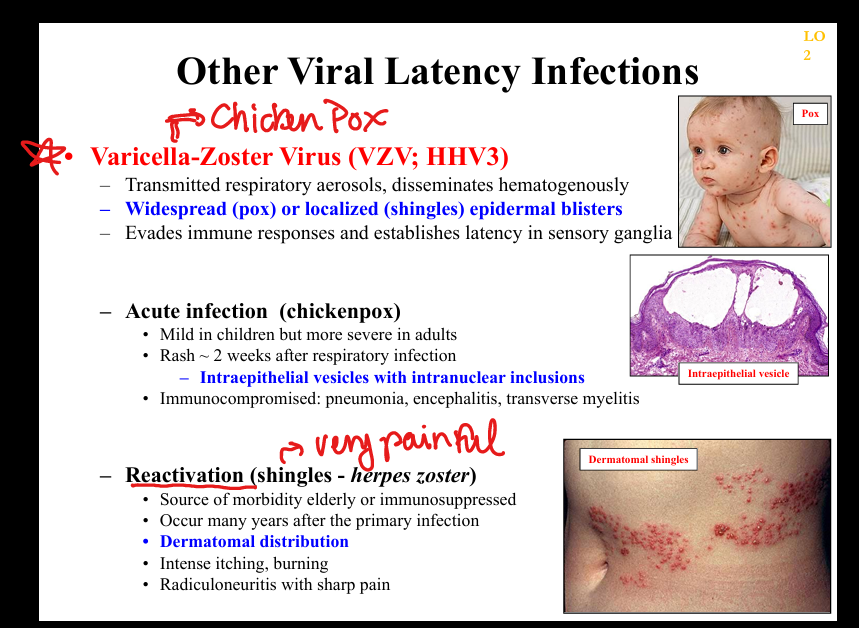

What clinicopathologic changes occur in VZV infection?
Chickenpox: diffuse vesicles, pneumonia, encephalitis; shingles: dermatomal vesicles, radiculoneuritis, post‑herpetic neuralgia; histology shows intraepidermal vesicles with inclusions.

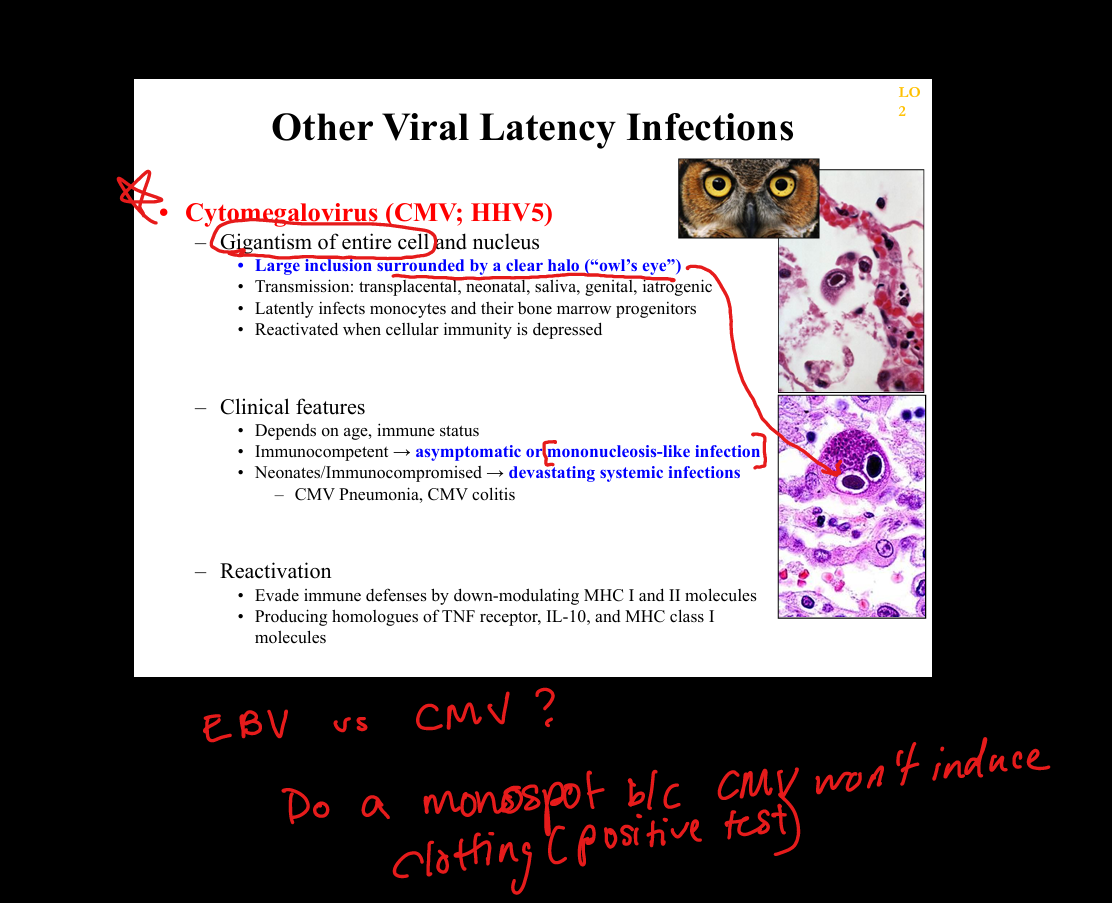
What is the pathogenesis of CMV (Cytomegalovirus) latency?
Primary infection(mono like illness), giganticism of entire cell → latency in monocytes and bone marrow progenitors → reactivation with immunosuppression; CMV downregulates MHC I/II and produces IL‑10 homologues. Owl Eye and giant monocytes
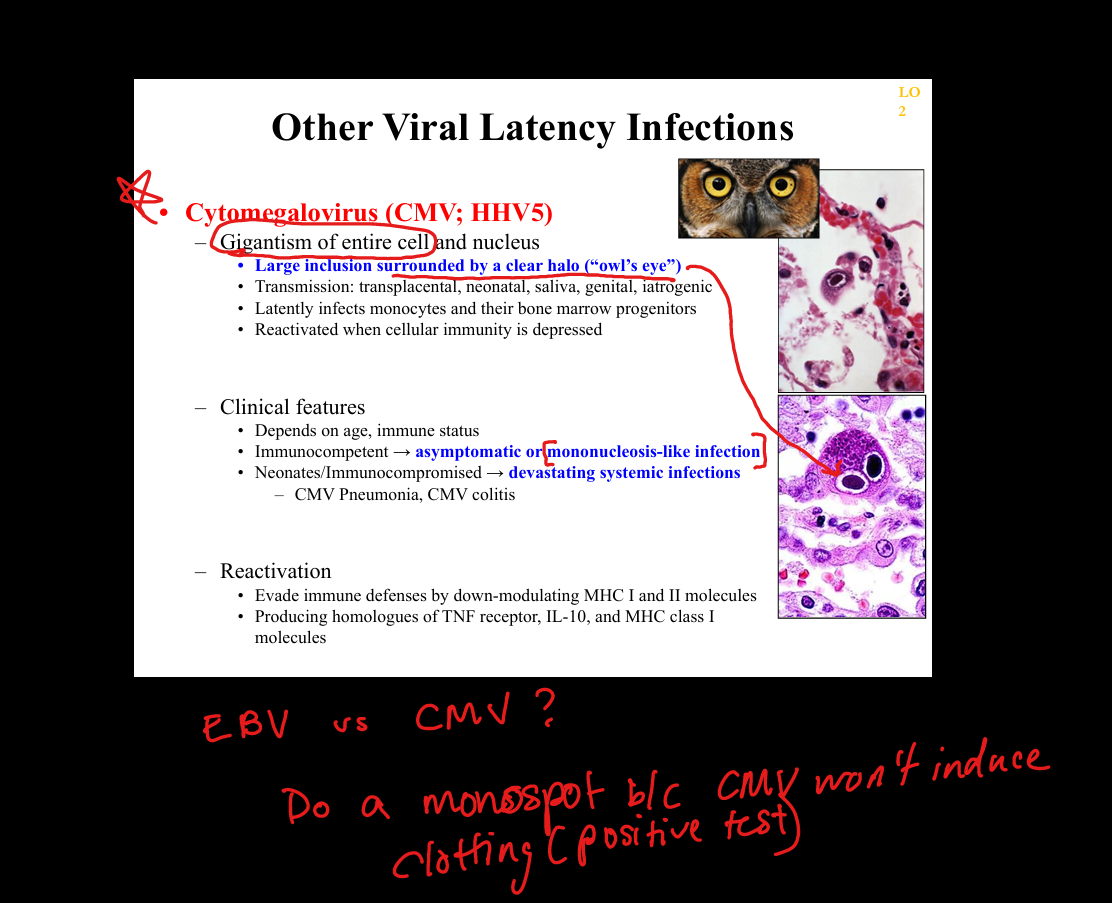

What clinicopathologic changes occur in CMV infection?
Immunocompetent: mono‑like illness; immunocompromised: CMV colitis, retinitis (“pizza pie”), pneumonitis; histology shows large cells with “owl’s eye” inclusions.

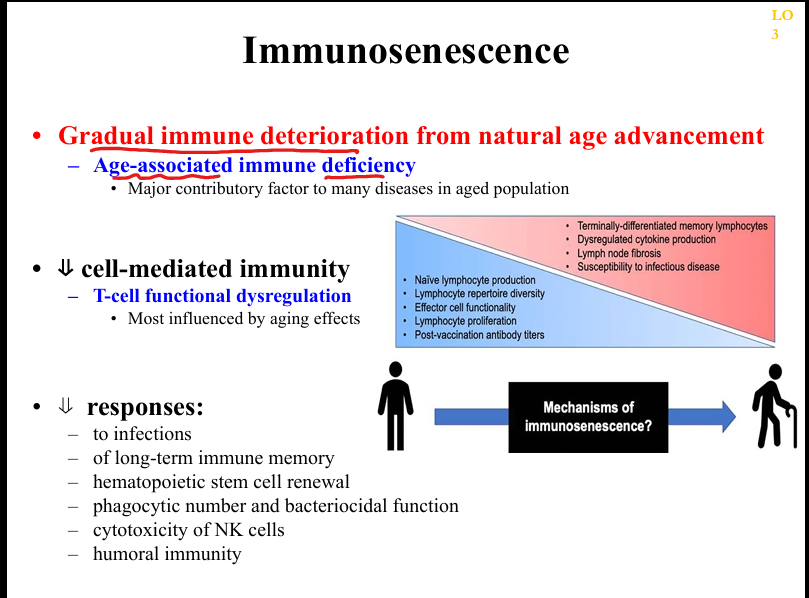
What mechanisms drive immunosenescence?
Thymic involution, chronic antigenic stimulation (e.g., CMV), stem cell exhaustion, lymph node fibrosis, reduced repertoire diversity, impaired phagocyte function.

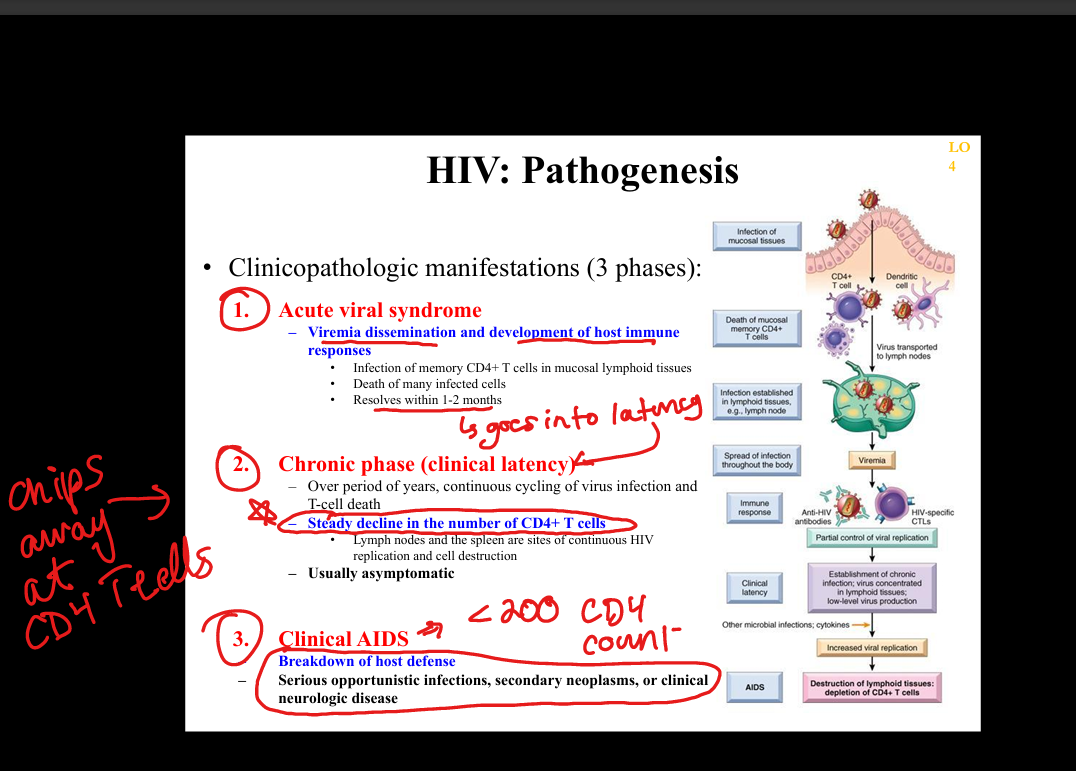
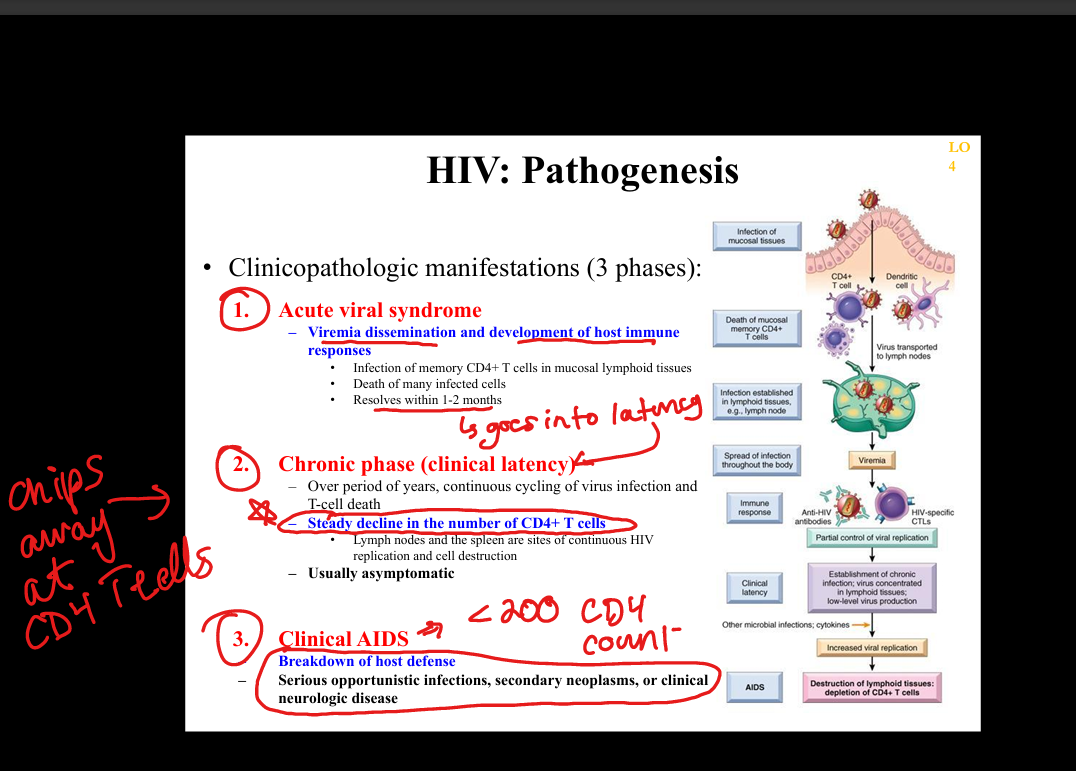
What is the natural history of HIV infection?
Acute HIV syndrome → clinical latency (persistent replication in lymphoid tissue) → progressive CD4 decline → AIDS (CD4 <200 or AIDS‑defining illness).
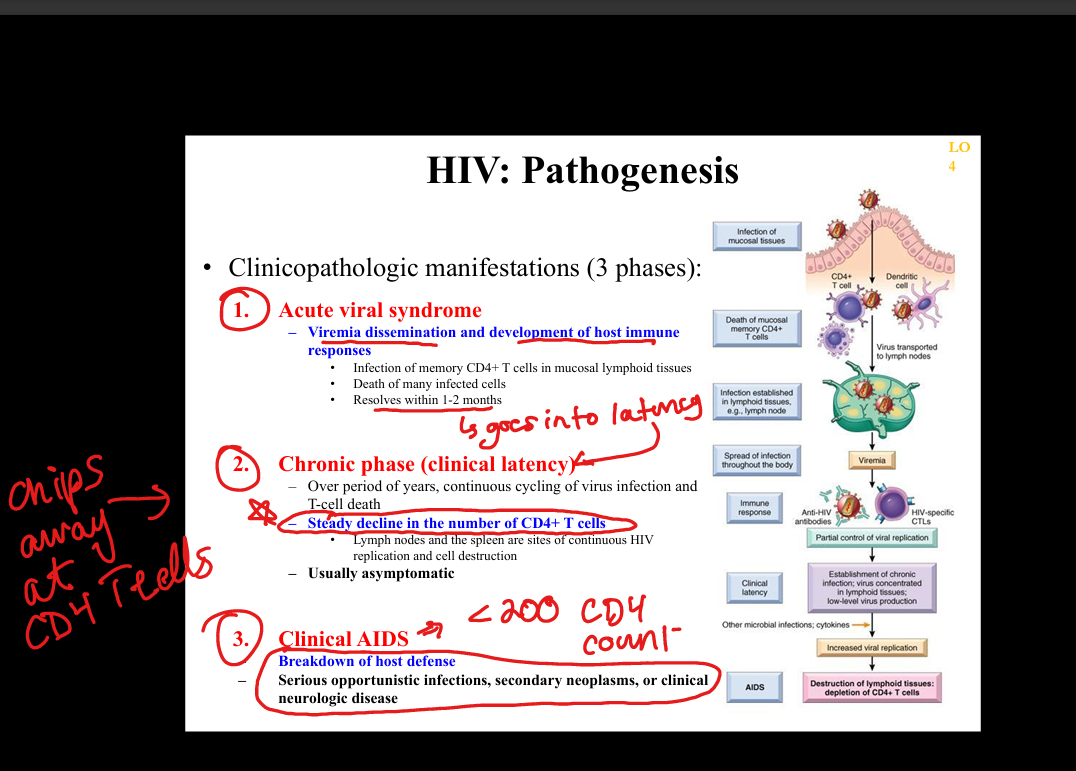

What is the pathogenesis of HIV infection?
HIV infects CD4+ T cells, macrophages, dendritic cells via gp120 binding to CD4 + CCR5/CXCR4 → integration into host genome → chronic immune activation → CD4 depletion → immunodeficiency.

What are the major immune abnormalities in HIV?
CD4+ T‑cell loss, impaired macrophage/dendritic function, B‑cell hyperactivation, loss of mucosal immunity, chronic inflammation, immune exhaustion.

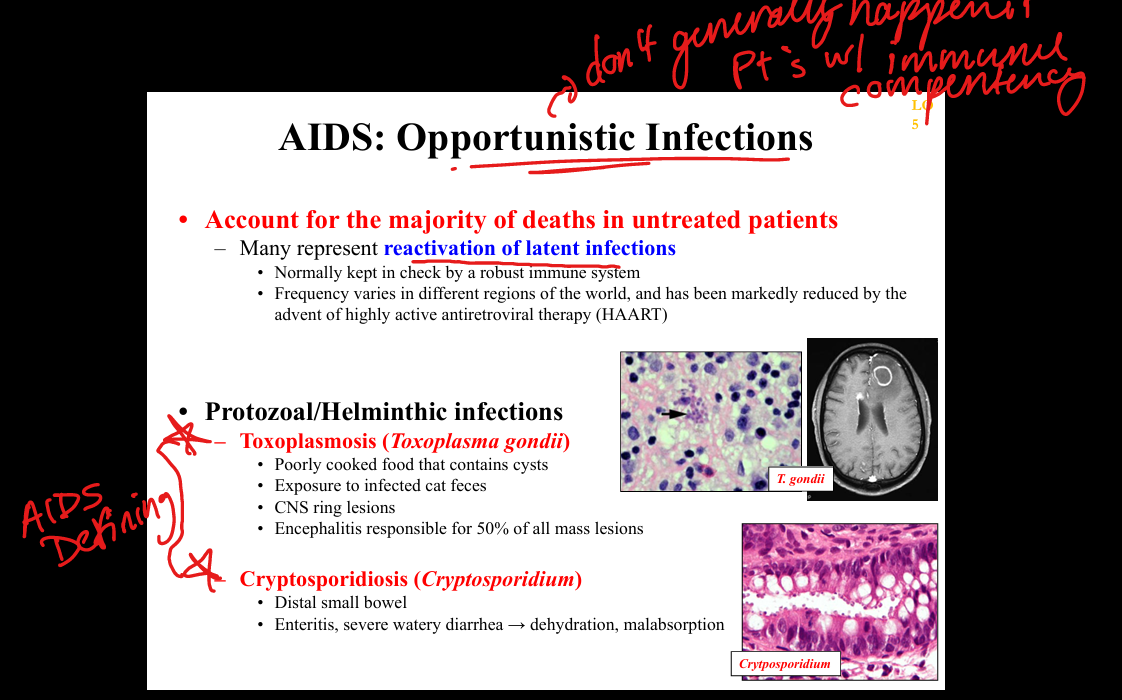
What protozoal opportunistic infections occur in AIDS?
Toxoplasma gondii (ring‑enhancing brain lesions), Cryptosporidium (chronic watery diarrhea), Isospora (diarrhea).

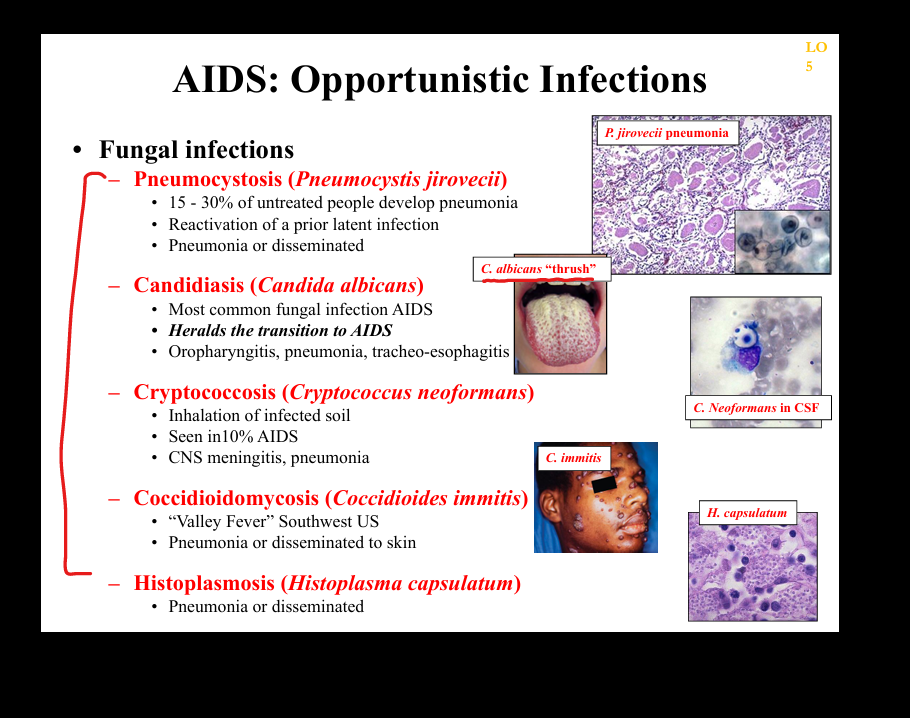
What fungal opportunistic infections occur in AIDS?
Pneumocystis jirovecii pneumonia (PCP), Cryptococcus neoformans meningitis, Histoplasma and Coccidioides disseminated infections, Candida esophagitis.

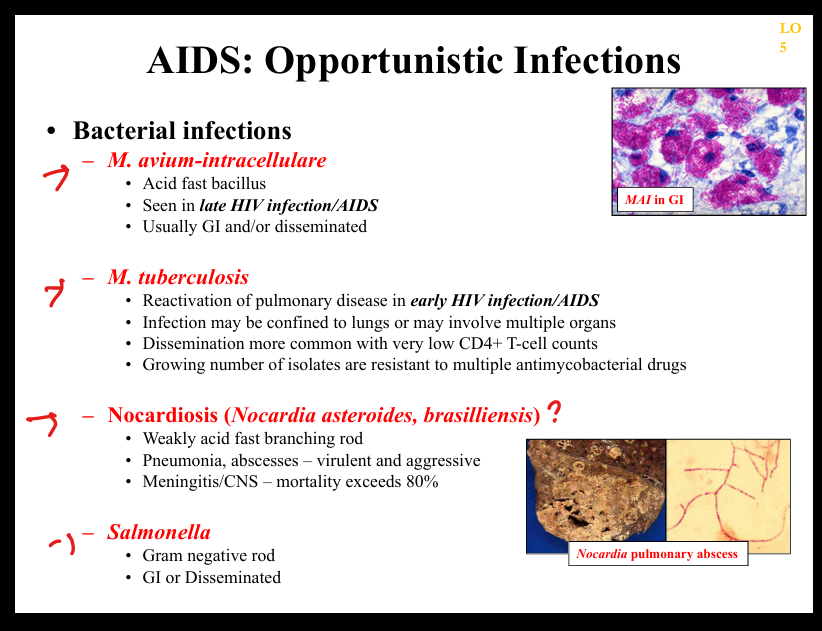
What bacterial opportunistic infections occur in AIDS?
Mycobacterium avium complex (MAC), Mycobacterium tuberculosis, Salmonella bacteremia, Listeria, typical pyogenic bacteria.

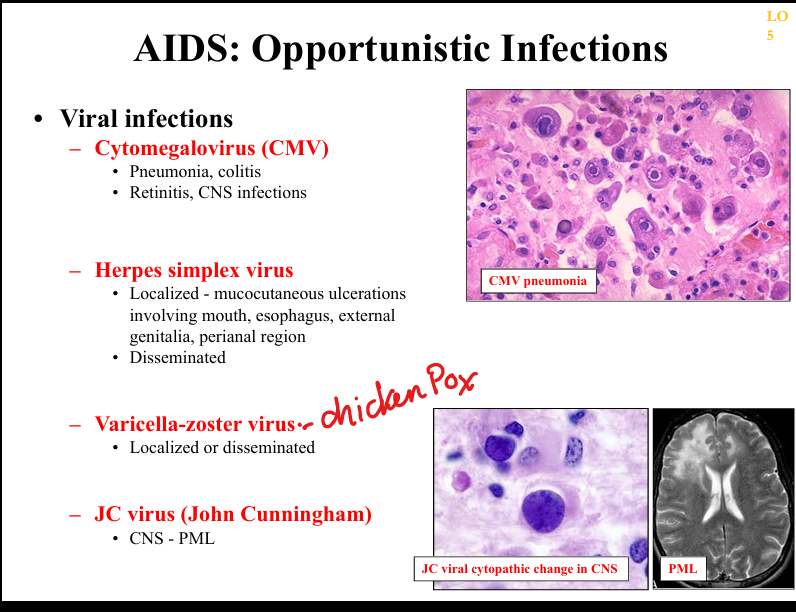
What viral opportunistic infections occur in AIDS?
CMV retinitis/colitis, HSV chronic ulcers, VZV disseminated infection, JC virus (PML), EBV‑associated lymphomas.

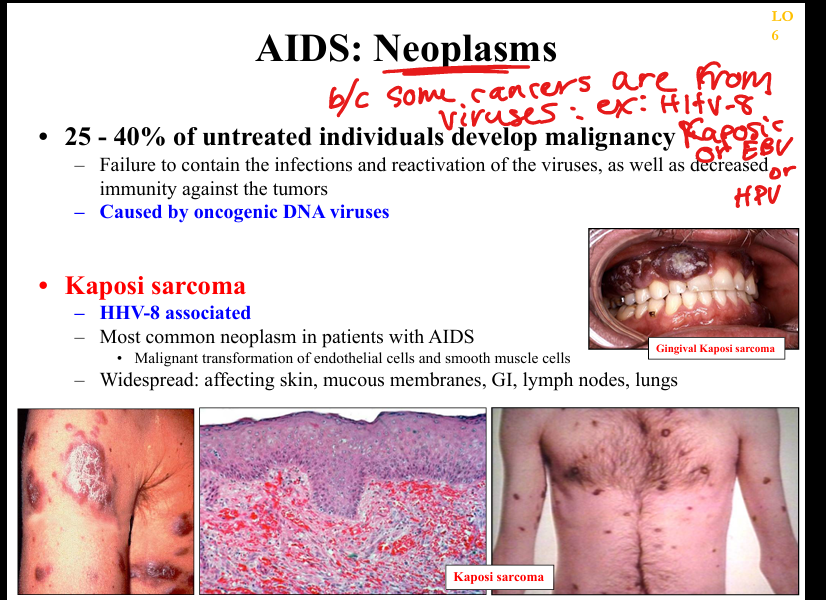
What is the pathogenesis of Kaposi sarcoma in AIDS?
HHV‑8 infection of endothelial cells → viral oncogenes promote angiogenesis and proliferation → violaceous skin lesions; worsens with immunosuppression.

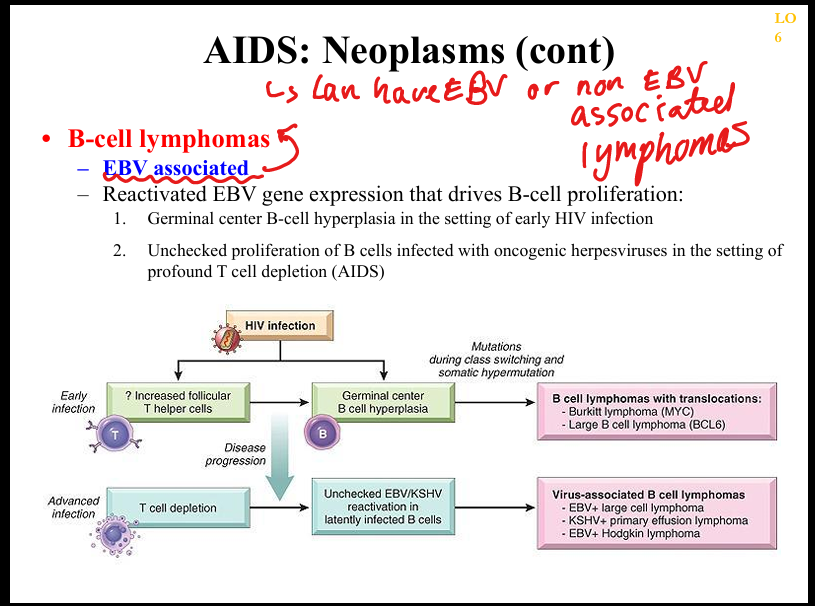
What is the pathogenesis of AIDS‑related B‑cell lymphomas?
EBV‑driven proliferation of B cells in setting of impaired T‑cell surveillance → DLBCL, primary CNS lymphoma, primary effusion lymphoma.

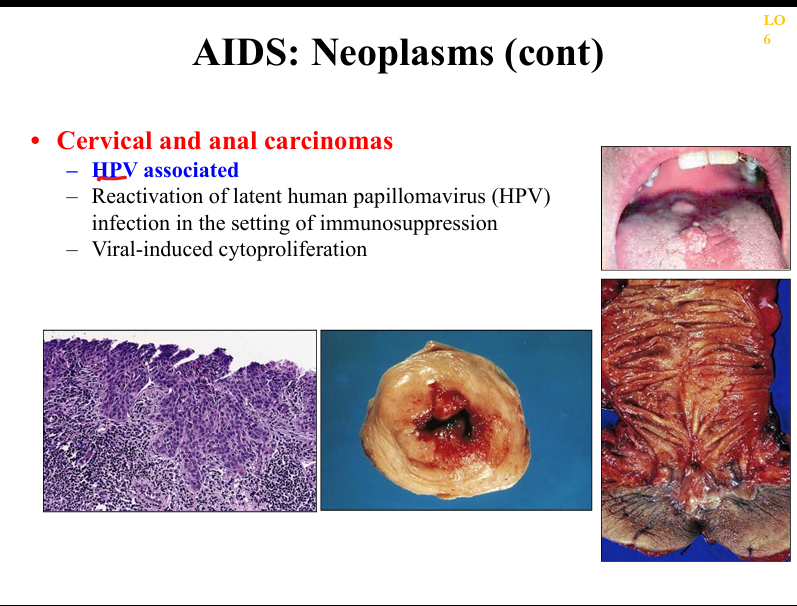
What is the pathogenesis of cervical/anal carcinoma in AIDS?
High‑risk HPV infection (16/18) with impaired immune clearance → E6/E7 inactivation of p53/Rb → dysplasia → carcinoma; incidence increased in HIV.

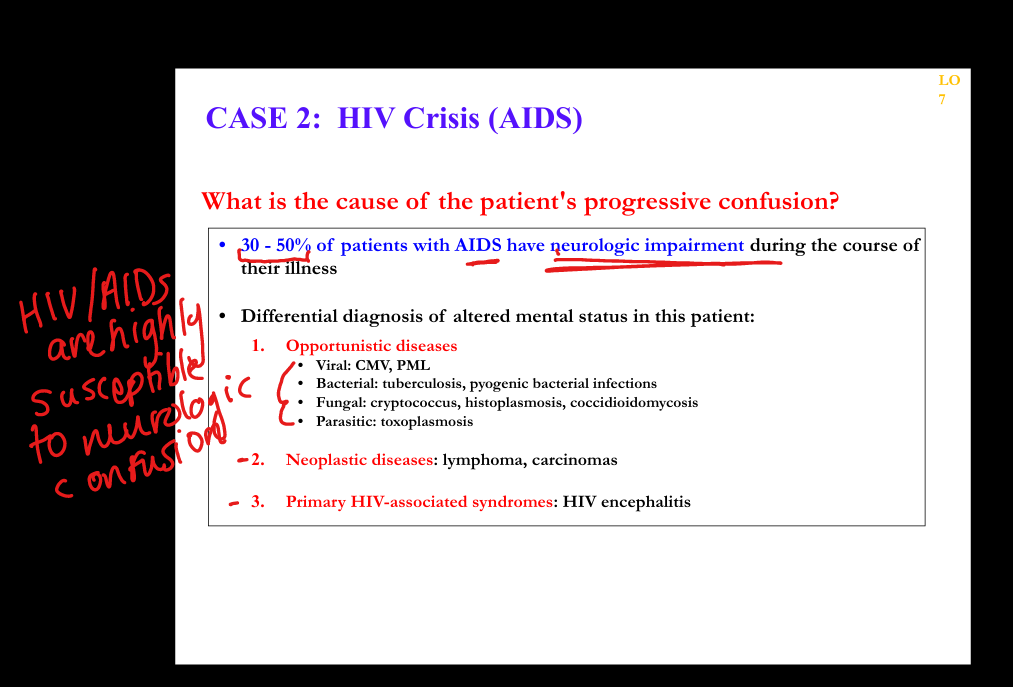
What neurological manifestations occur in AIDS?
HIV‑associated neurocognitive disorder (HIV encephalitis), PML (JC virus), CMV encephalitis, toxoplasmosis, peripheral neuropathy, vacuolar myelopathy.
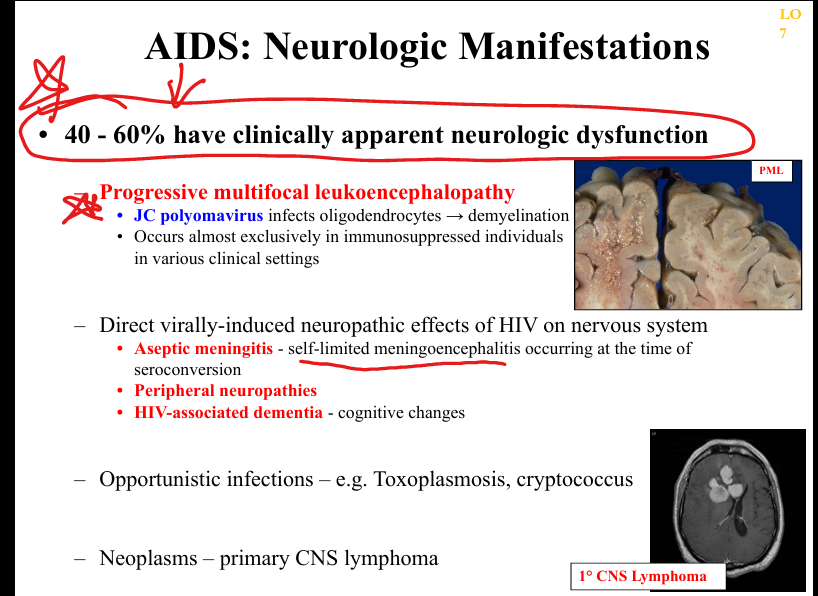

What are the clinicopathologic features of HIV encephalitis?
Microglial nodules, multinucleated giant cells, white matter pallor, cognitive decline, behavioral changes, motor deficits.