Atomic Structure
1/48
Earn XP
Description and Tags
BI1014 Biological Chemistry
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
Pure substance
matter that has a fixed or definite composition
can be elements or chemical compounds
Element
simplest type of material made up of atoms
Compound
pure substance consisting of atoms of two or more elements always chemically combined in the same proportion
Mixtures
two or more different substances are physically mixed, but not chemically combined together
Homogenous mixture
composition is uniform throughout the sample
Heterogenous mixture
components do not have a uniform composition throughout the mixture
Conservation of Mass
mass cannot be created or destroyed; it simply changes forms.
Law of Definite Proportions
given chemical compound always contains the same elements in the exact same proportion by mass
Law of Multiple Proportions
whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers
Atomic Structure
Nucleus and shell
Nucleus is positively charged and consists of protons and neutrons
Nucleus contains 99.9% of mass
Shell negatively charged contains electrons
Shell 100,000 times larger than nucleus
Proton
Symbol
Charge
Mass (amu)
Location in atom
p or p+
+ 1
1.007
Nucleus
Neutron
Symbol
Charge
Mass (amu)
Location in atom
n or n0
0
1.008
Nucleus
Electron
Symbol
Charge
Mass (amu)
Location in atom
e-
- 1
0.00055
Outside nucleus
Atomic number (Z)
number of protons in an atom which is equal to number of electrons
Mass number (A)
number of protons + number of neutrons
Isotopes
atoms of the same element that have the same atomic number but different number of neutrons
Avogadro’s Number
6.02 ×10²³
Number of atoms in one mole of any element
Number of atoms in 12g of 12C
Molar mass
quantity in grams that equals the atomic mass of that element/compound
Molar mass equation
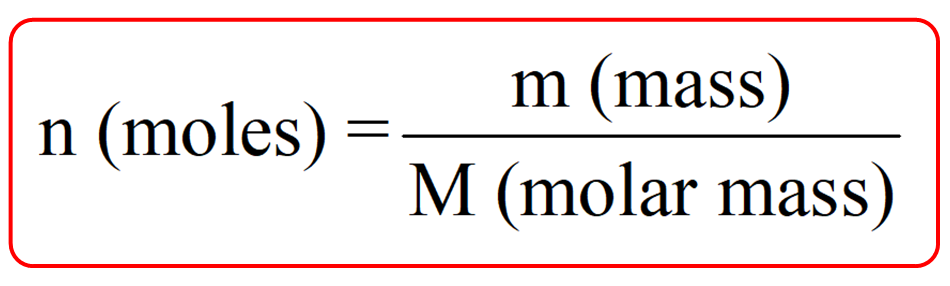
Electronegativity
Electronegativity is a measure of the degree to which an atom ‘draws’ electrons towards itself in a bond
The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Electronegativity is related to ionisation energy.
Atoms with low ionisation energies have low electronegativities, because their nuclei do not exert a strong attractive force on the electrons, and vice versa.
In a period, moving from left to right, electronegativity increases (as ionisation energy increases).
Going down a group in the periodic table, electronegativity decreases as both atomic number and atomic radius increase, and ionisation energy decreases.
Ionisation energy
Ionisation energy: energy required to completely remove an electron from a gaseous atom or ion
The closer and more tightly bound an electron is to the nucleus, the more difficult it will be to remove.
Ionisation energies increase moving from left to right across a period (decreasing atomic radius), and decrease moving down a group (increasing atomic radius).
Group 1 elements have low ionisation energies, with the loss of an electron forming a stable octet.
Electron affinity
Electron affinity: ability of an atom to accept an electron
Atoms with stronger effective nuclear charge have greater electron affinity.
For example, Group 17 elements, the halogens, have high electron affinities; the addition of an electron to such elements results in a completely filled outer electron energy level.
ED
Electron energy levels
Regions around an atom’s nucleus where electrons are found, with each level corresponding to a specific energy state.
Main shells
Discrete energy levels
Main shells 1, 2, 3, 4, 5, 6, 7,
Each shell contains maximal 2 n2 electrons (n = main shell number)
Subshells
Main shells divided into subshells: s, p, d, f
Each subshell contains a maximal number of electrons
S: 2 electrons
P: 6 electrons
D: 10 electrons
F: 14 electrons
The higher the main shell, the more subshells
N = 1 only 1 s = 2 electrons
N = 2: one s and one p subshell = 2 + 6 = 8 electrons
N = 3: one s, one p and one d subshell = 2 + 6 + 10 = 18
Atomic orbitals
regions in space where there is a high probability of finding electrons
Size reflects 95% probability of presence of electrons
Number of nodes dependent on main shell n and defines kind of subshell
Number of subshells reflect possible spatial orientation
Atomic orbitals have particular shapes and can hold a maximum of two electrons. Closest to the nucleus is the lowest energy level with the value n = 1
Energy level of electrons
Characterised by 4 quantum numbers
Electrons differ in at least one number
Principle quantum number n (main shell): size of orbital(s)
Angular quantum number I (subshell): shape of orbital
Magnetic quantum number m (no of orbitals in subshell): orientation of orbital
Spin quantum number s
'spin' of electron within orbital
Values -1/2 and + 1/2
Maximum of 2 electrons per orbitals
Group number
represents valence electrons
Period number
represents number of electron shells
Subshells in periodic table
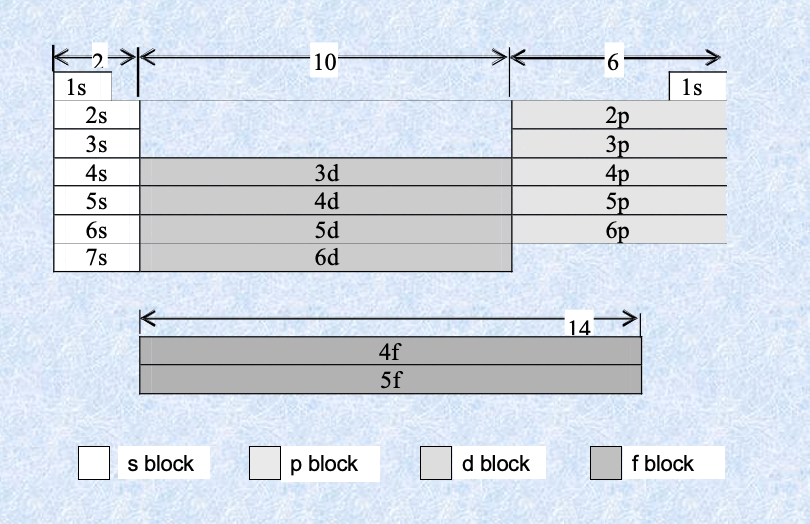
Orbital filling order
Electrons fill orbitals of the lowest energy first. So the first level (n = 1) with its 1s orbital is filled before an electron can occupy the second level.
Within an energy level electrons fill the orbitals with the lowest energy first. s orbitals represent a lower energy configuration than p orbitals and so electrons will fill the s orbital within an energy level before filling the p orbitals.
Individual orbitals can hold a maximum of two electrons. Each s orbital (1s, 2s, 3s etc.) can only accommodate two electrons at the most. When two electrons occupy the same orbital they spin in opposite directions, otherwise their negative charges would force them apart.
Electron configuration in boxes
Each box represents an individual atomic orbital, and arrows (representing electrons) are used to show the specific placement of electrons within those orbitals.
Electrons fill lower energy orbitals first, no two electrons in the same orbital can have the same spin and every orbital in a sub shell is singly occupied before any are double occupied
Summary of Trends in Periodic Properties of Representative Elements
Period Properties - Valence electrons, Atomic size, Ionisation energy, Metallic character
top to bottom within a group
left to right across a period
Valence electrons
Remains the same
Increases
Atomic size
Increases due to the increase in number of energy levels
Decreases due to the increase of protons in the nucleus that pull electrons closer
Ionisation energy
Decreases because valence electrons are easier to remove when they are farther from the nucleus
Increases as the attraction of the protons for outer electrons requires more energy to remove an electron
Metallic character
Increases because valence electrons are easier to remove when they are farther from the nucleus
Decreases as the attraction of the protons makes it more difficult to remove an electron
Stable isotopes
Isotopes of an element that do not undergo radioactive decay and remain unchanged over time. Most naturally occurring isotopes of elements up to atomic number 19 have stable nuclei.
Radioactive isotopes
Elements with unstable nuclei due to uneven ratio of neutrons and protons which the nuclear forces cannot offset the repulsions between the protons. It spontaneously emits small particles called radiation to become more stable.
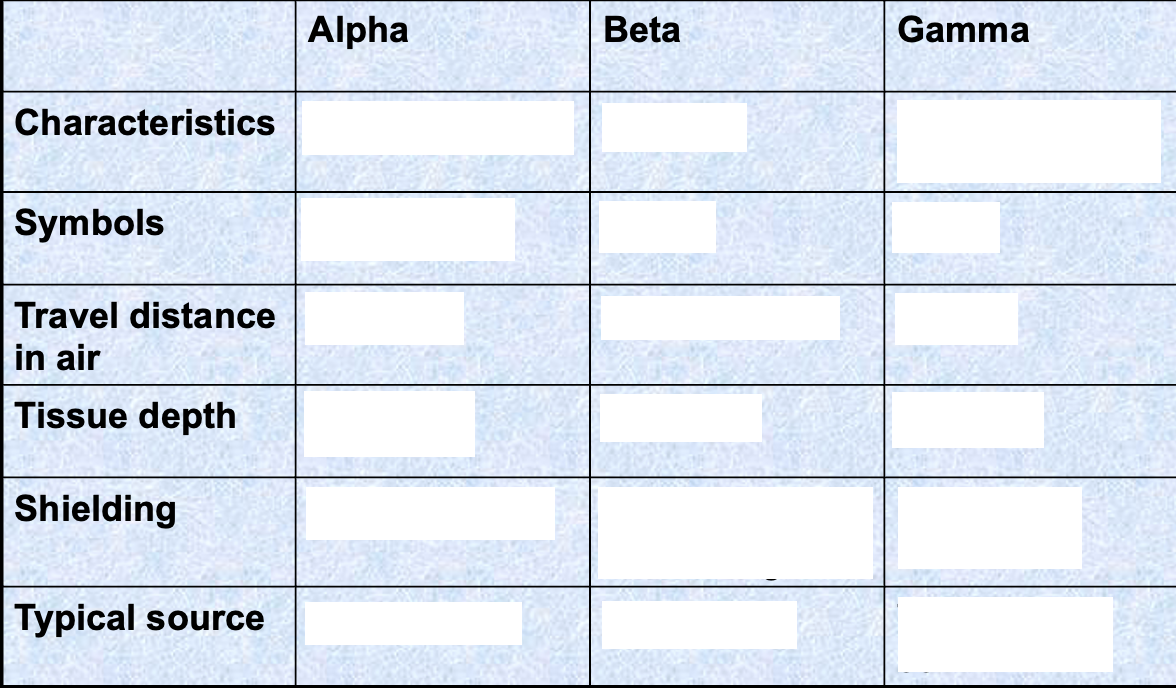
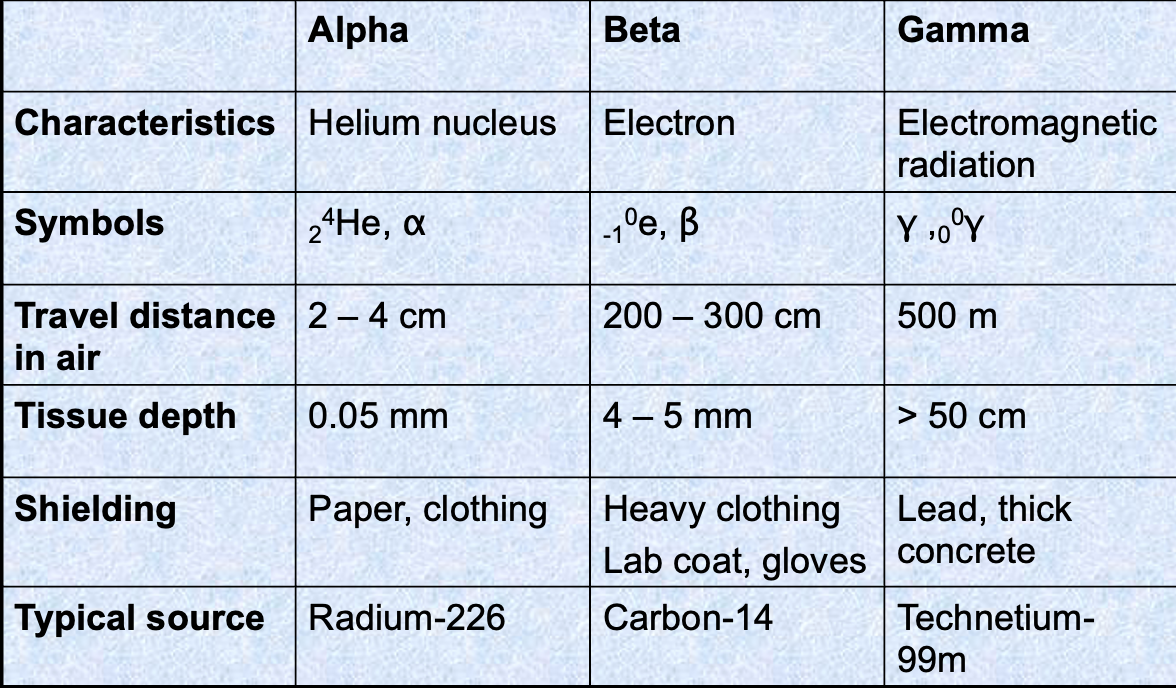
Alpha radiation features
Emitted by heavy nuclei (elements)
Helium nucleus
2 protons and 2 neutrons
Mass number of 4
Atomic number of 2
Charge of 2+
α symbol

Beta radiation features
Emitted by light and heavy nuclei
High energy electron
Breakdown of a neutron into a proton and an electron
Mass number of 0
Atomic number of -1
Charge of -1
β symbol
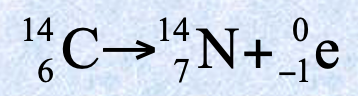
Gamma radiation features
Excited nuclei and excess energy
Released when an unstable nucleus undergoes a rearrangement of its particles to give a more stable, lower energy nucleus
Mass number of 0
Atomic number of 0
Charge of 0
γ symbol
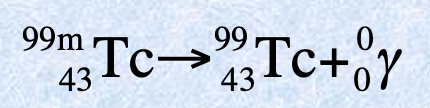
Positron features
Antimatter of an electron
Proton in an unstable nucleus is converted to a neutron and positron. The neutron remains in the nucleus, but the positron is emitted from the nucleus.
Mass number of 0
Atomic number of 1
Charge of 1+
β + symbol
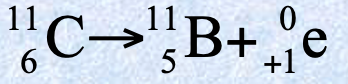
Orbital electron capture features
One of the orbital electrons is captured by a proton in the nucleus, this creates a neutron and a neutrino which is emitted
Electron is removed so atomic number decreases by 1
ν e symbol
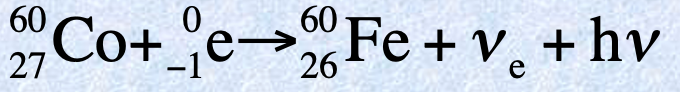
Measures of radiation
Activity: number of nuclear disintegrations per second - becquerel (Bq) which is 1 disintegration/s
Old unit: curie (Ci) number of disintegrations that occur in 1s for 1g of radium
Absorbed radiation: amount of radiation absorbed by a gram of material - Gray 1J/kg = 1 Gy
Old unit: radiation absorbed dose 1 rad = 0.01 Gy
Rem (radiation equivalent in humans): measures the biological damage - New unit: Sievert 1 Gy x factor = 1 Sv
Old unit: radiation equivalent in humans 1 rem = 1 rad x factor
Factors:
1 for Beta, Gamma and X-rays
10 for high-energy protons and neutrons
20 for alpha radiation
Half life
Amount of time it takes for one half of a sample to decay
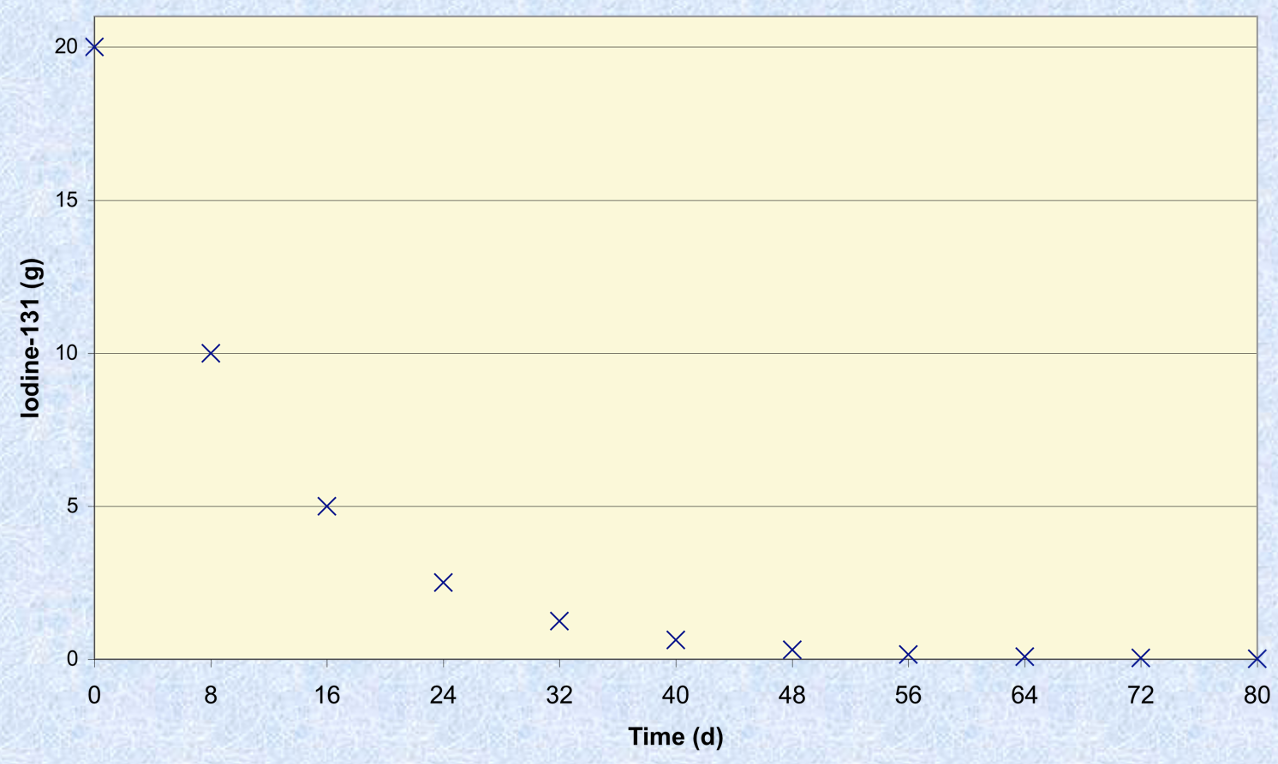
Natural radioisotopes
Natural radioisotopes
Isotopes occurring in nature that are unstable and undergo radioactive decay. They have a long half-life
Artificial radioisotopes
Isotopes that are artificially created, are typically unstable and undergo radioactive decay, emitting radiation.
Applications of radioactivity
Carbon dating
Radio-immuno assay
Medical
Visualisation
Treatment
Energy generation
Fission
Fusion
Medical applications using radioactivity
Radioisotopes go to specific sites in the body
By detecting the radiation they emit, an evaluation can be made about the location and extent of an injury, disease, tumour, or the level of function of a particular organ
Higher levels of radiation are used to treat or destroy tumours
Nuclear fission
The bombardment of a large nucleus breaks it apart into smaller nuclei, releasing one or more types of radiation and a great amount of energy.
Nuclear fusion
Small nuclei combine to form larger nuclei while great amounts of energy are released