Clinical Cancer Genetics lecture
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
What is cancer?
a disease in which abnormal cells divide without control and ne can invade and colonise nearby tissues
What is a neoplasm?
new growth
Another word for neoplasm?
tumour
what are the 2 things cells are doing to form a tumour or neoplasm?
an abnormal cell that grows (increase in mass) and proliferates (divides), out of control will give rise to an abnormal mass of tissue called a tumour or neoplasm
what is benign?
the neoplastic cell have not yet become invasive
What is malignant?
when the cell have acquired the ability to invade surrounding tissue
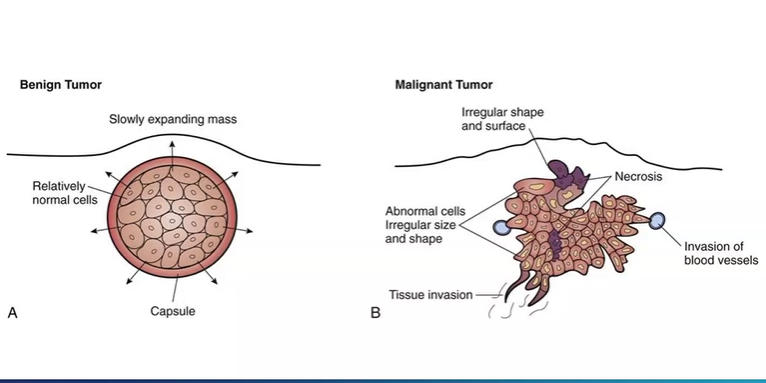
compare benign and malignant tumour
slow expanding mass , normal cells , proliferation than normal cells surrounding, often capsulated , smooth surface
more irregular shape, cells of different shapes and sizes, some cells die as not enough oxygen, some cells begin to invade nearby tissues
What is a mutation?
a change in the DNA sequence of an organism
list 3 reasons for mutations?
errors in DNA replication during cell division
exposure to mutagens (agents that cause changes in organisms genetic material including, tobacco products, UV radiation, chemicals)
viral infection
mutations are the ultimate source of what?
all genetic variation, providing the raw materials on which evolutionary forces such as natural selection can act
germline mutations vs somatic mutations?
where do they occur?
can they passed onto offspring?
germline mutations occur in eggs and sperm - can be passed onto offspring
somatic mutations - occur in body cells - are not passed onto offspring
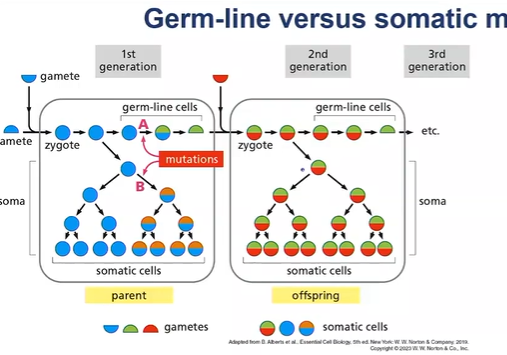
most cancer related mutations occur in which cells, somatic or germ line?
somatic cells
therefore most cancers are not linked to inherited faulty genes, only about 5% of daignosed cancers are linked to an inherited faulty gene
Mutant germ line alleles of 2 examples may lead to which cancers?
BRCA1 and 2 confer an inborn susceptibility to breast and ovarian cancer
around 70% of women with faulty BRCA 1 and 2 will go on to develop breast cancer by age 80
BRCA genes are important in DNA repair
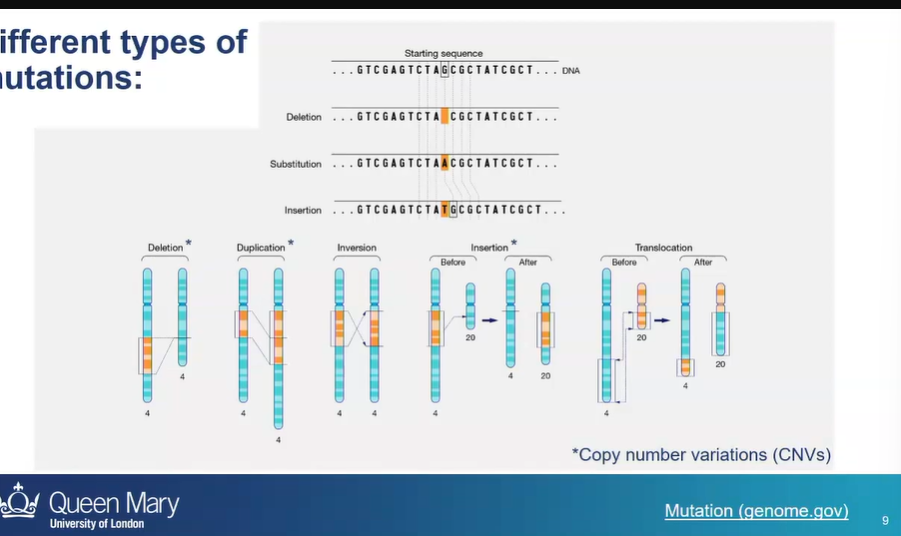
what are 2 broad groups of DNA mutations?
mutations that affect a single nucleotide
or mutations that affect a large stretch if chromosomal DNA
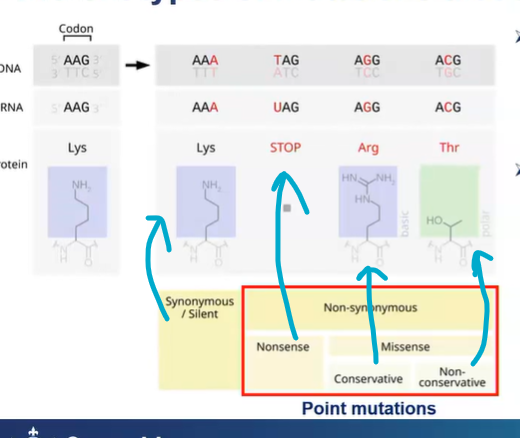
Point mutations: mutation that affect a single nucleotide
what are the different outcomes/types of point mutations?
synonymous/silent mutations - is he substitution of one nucleotide for another in an exon of a gene coding for a protein, such that the produced amino acid sequence is not modified
or it affects the non coding DNA - silent mutations
non-synonymous - arginine and lysine have basic chemical function, they’re both basic - conservative
non-conservative - Thr is a polar amino acid - different chemical acid so its different to arg
nonsense - stop codon so ribosome stops translating and therefore a shorter polypeptide
How can the cells protect against nonsense mutations?
nonsense-mediated mRNA decay
What is a frame shift mutation?
A single nucleotide is inserted into the DNA sequence, so downstream to the inserted nucleotide there will be change in the reading frame so different amino acids along the rest of the frame
Also a different stop codon appears so a truncated protein is produced
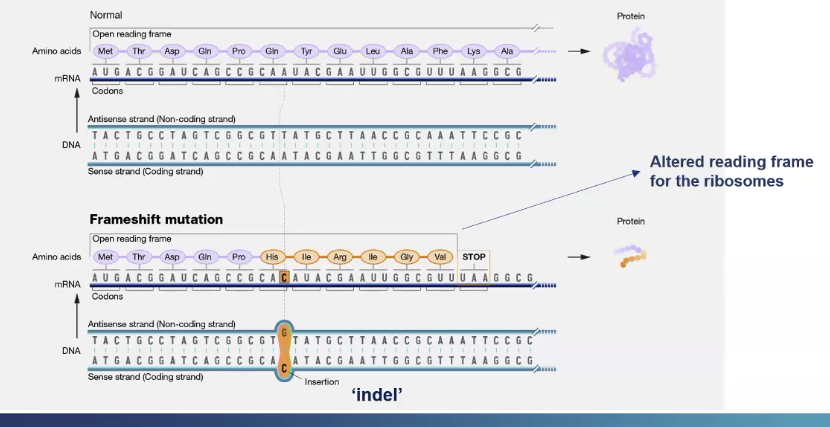
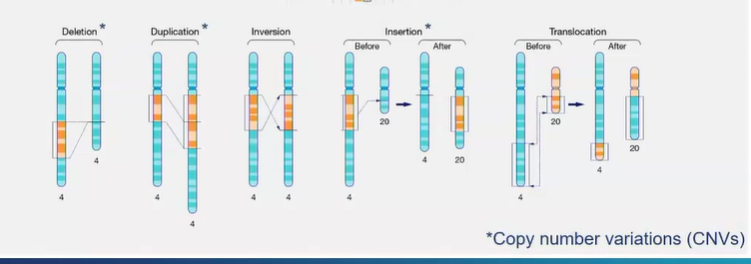
what are 5 types of mutations that can occur on chromosomes?
deletion
duplication - a fragment of DNA on a chromosome is duplicated
inversion - orientation of DNA sequence is flipped
insertion - a piece of chromosomal DNA is cut out of one chromosome and inserted into another
translocation - reciprocal exchange of chromosomal NA between 2 chromosomes
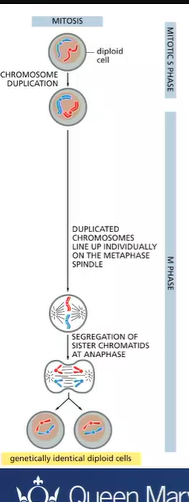
what happens in mitosis?
the maternal and paternal homologous chromosomes are equally distributed to generate identical daughter cells
mistakes during mitosis can lead to a special type of mutation called?
loss of heterozygosity - heterozygotes somatic cells become homozygous because one of the 2 alleles gets lost,
could get an exchange in DNA between maternal and paternal homologous chromosomes for an allele
so they are heterozygous for the rest of the alleles but homozygous for a specific allele
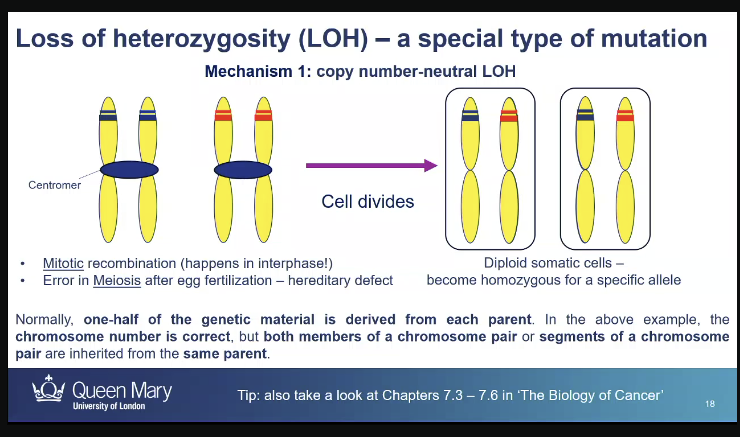
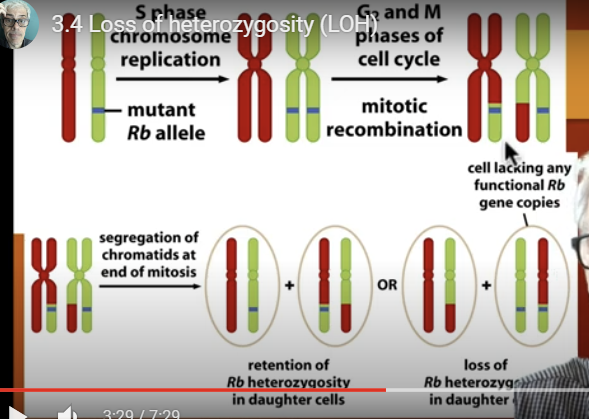
Loss of heterozygosity: what is another mechanism?
loss of one copy of a gene: hemizygosity which states of having unpaired genes in an otherwise diploid cell
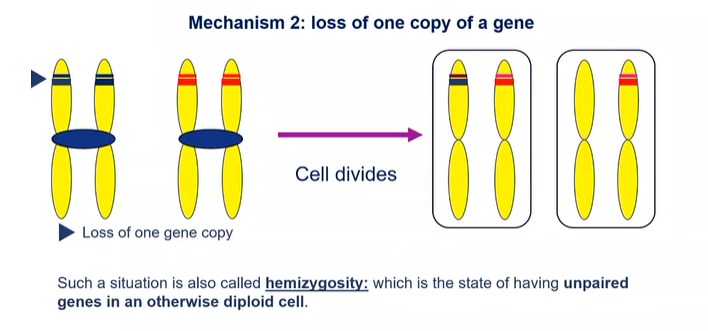
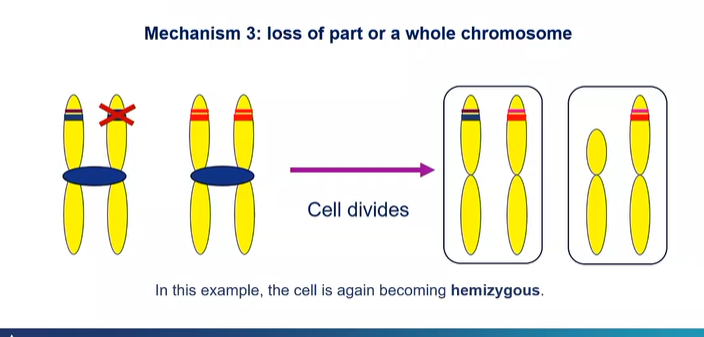
what is another mechanism in loss of heterozygosity?
An entire piece of chromosome gets lost
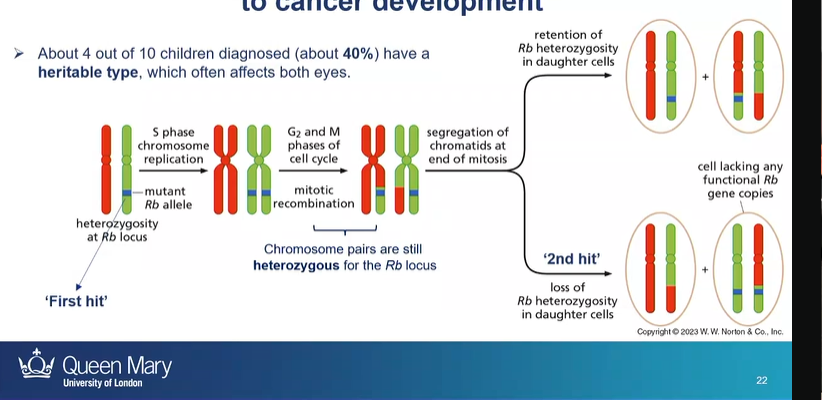
what cancer is an example of how LOH can contribute to cancer development?
retinoblastoma
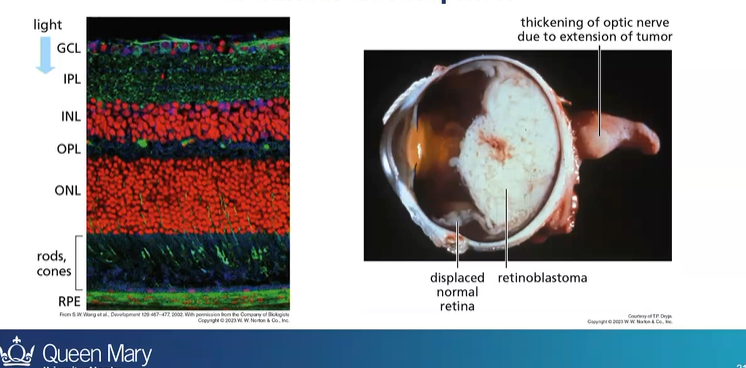
how does LOH lead to retinoblastoma
first hit - having a parent with a Rb mutated gene
then second hit is the loss of heterozygosity - due to mitotic recombination
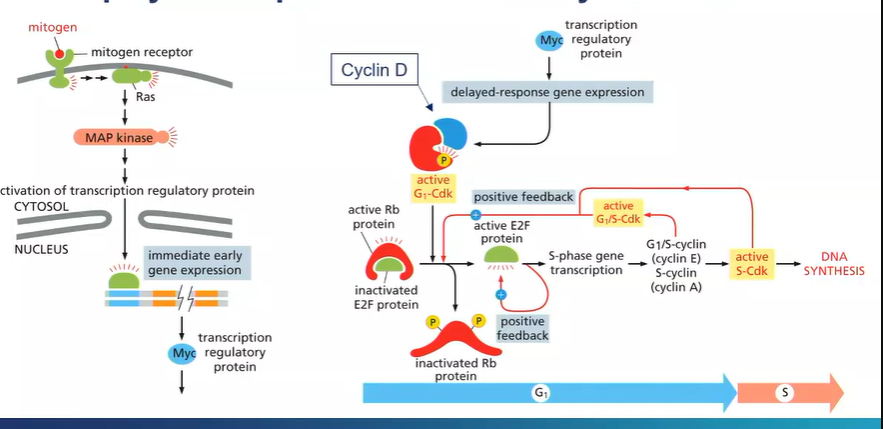
What is the signalling pathway that leads to retinoblastoma ?
mitogens - tell a cell to undergo mitosis
bind to mitogen receptors
induce a signalling cascade which cause changes to gene expression
genes are turned on in seconds/minutes
transcription factor - Myc
induced expression of other genes - cyclin D
binds to G1-Cdk - important regulators of the cell cycle
active G1-Cdk phosphorylates the Rb gene
the active form the Rb gene keeps the E2F protein in an inactive conformation
so mitogenic signalling presence, the Rb protein is phosphorylated so it becomes inactivated and the E2F is activated which leads to inducing of genes needed for cells to proceed from the G1 phase of the cell cycle to the s cycle
so mitogenic activation leads to continuation of DNA synthesis
causing retinoblastoma cells to proliferate continuously
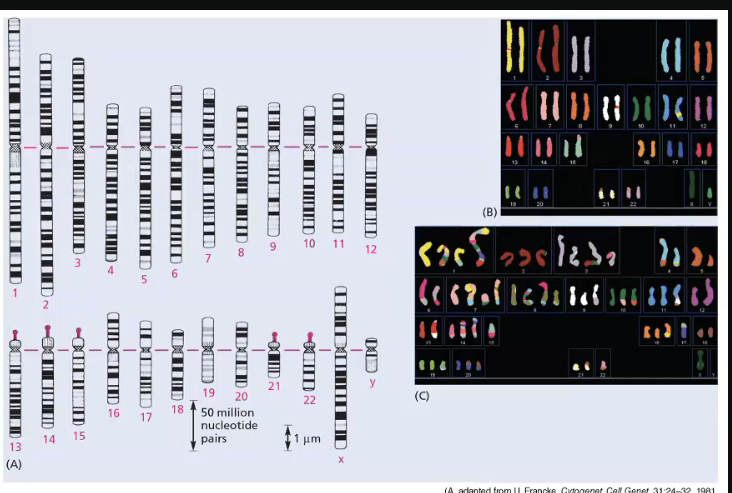
cancer cells typically show “chromosomal x”
chromosomal aberrations
includes changes in structure of individual chromosomes and in chromosome numbers - aneuploidy
the second diagram you can see segments of different colours, 2 chromosomes instead of 2 etc
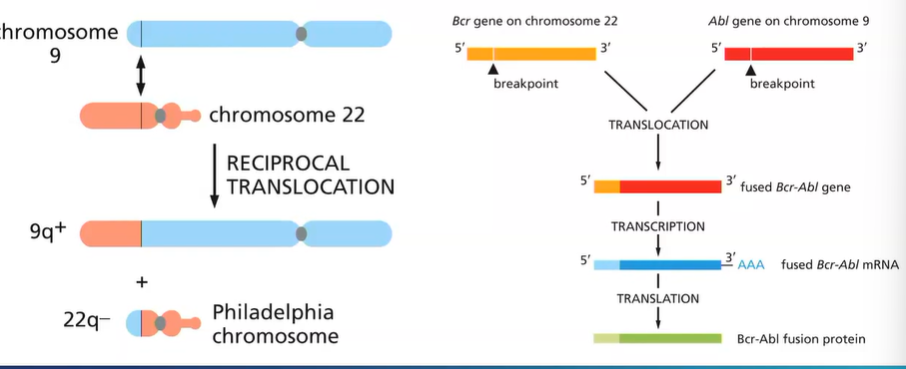
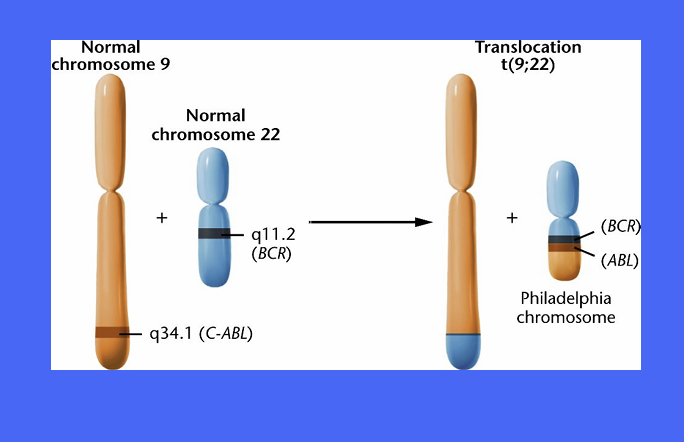
what chromosome mutation occurs in CML?
reciprocal translocation between chromosome 9 and 22
‘Philadelphia chromosome ‘ - small chromosome
Bcr-Abl fusion protein - active all the time - to undergo cell cycle
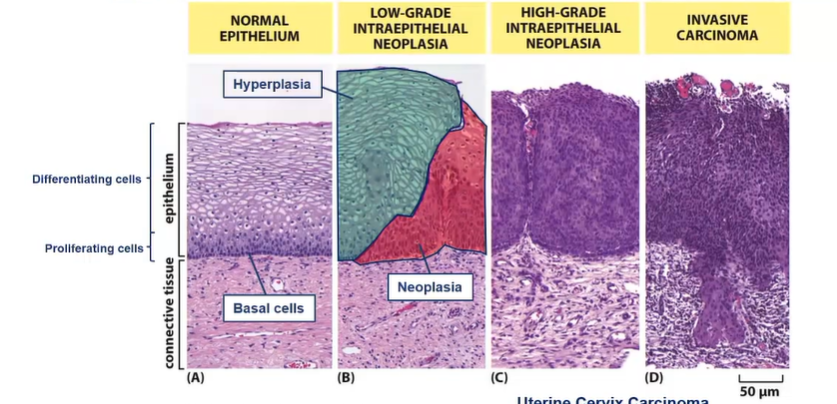
Histology provides evidence of which type of tumour formation process?
Multi-step process
accumulation of increasingly abnormal cells, driven by sequential accumulation of mutations
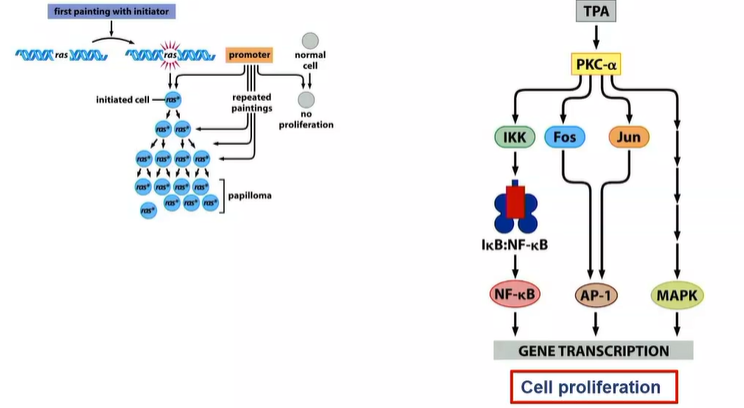
chemical model of tumour development requires 2 things?
promoter and initiator
forms benign tumour
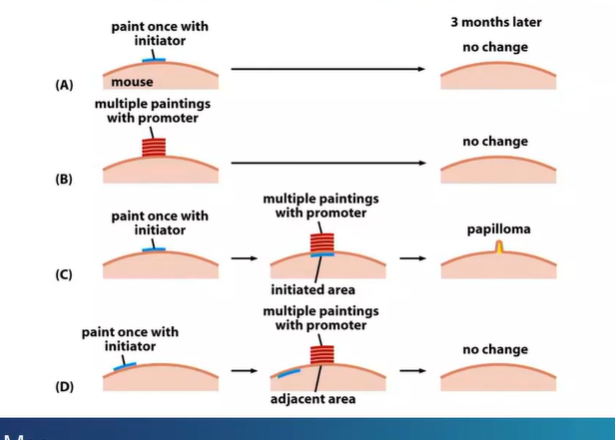
what can make this benign tumour become malignant?
further exposure to the initiator (chemical)
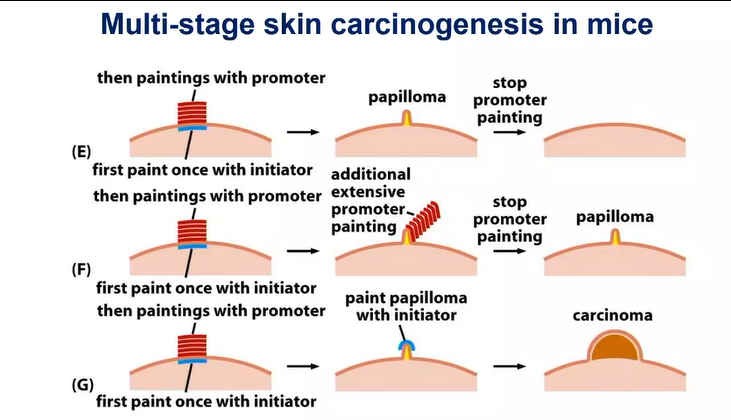
what are conclusions drawn up about initiators and promotors?
effects of tumour initiators are not reversible - causes genetic alteration
effects of tumour promoters are reversible - it exerts a nongenetic effect
cause mutations in Ras which keeps it active - keeps proliferation
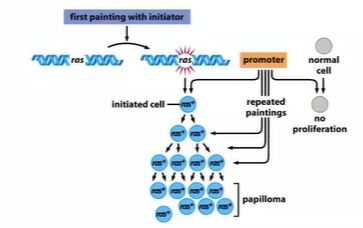
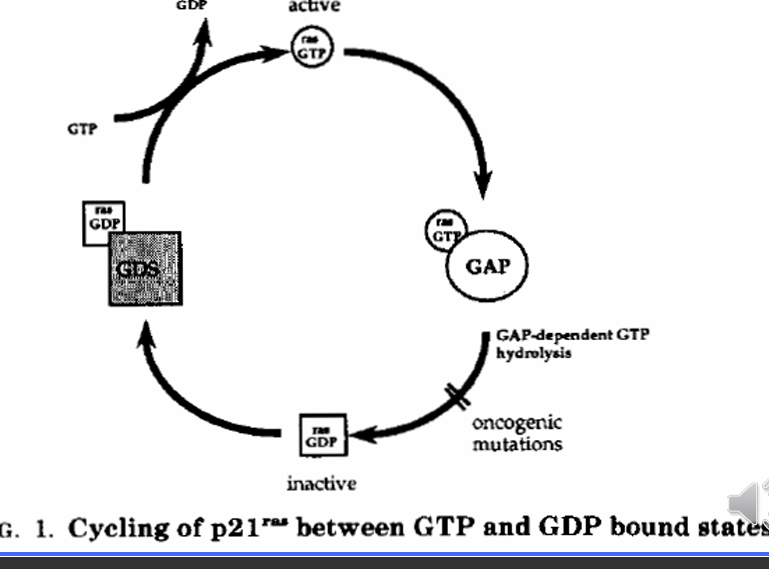
what important enzyme is a powerful oncogene in many cancers?
Ras - are GTPase
when mitogen signal binds to mitogen receptor
Ras protein becomes activated
activates a cascade - Raf, Mek, ERK, cascade (proteins and TF) - expression of MYC
mutations causes RAS to stay active so no mitogen is needed
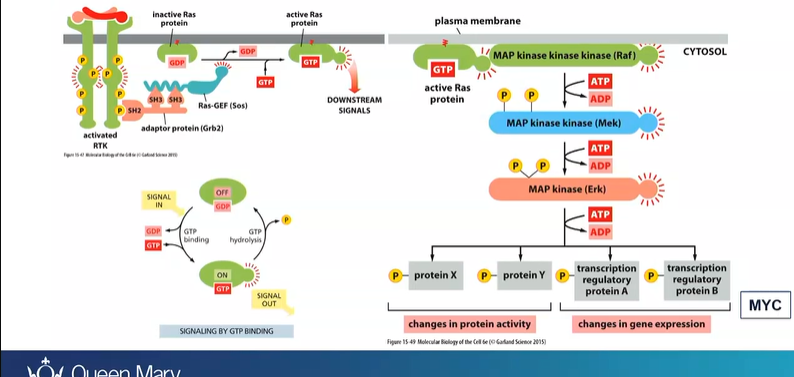
What does the promoter do if the initiator cause mutations in the Ras gene
RAS - proliferate slightly more frequently than those that don’t - so alone its not enough
to really proliferate - promoter TPA - activates several other signalling pathways that stimulate cell proliferation
Oncogenes usually originate as what?
proto-oncogenes
give an example of an oncogene
Ras
what are oncogenes?
normal and essential genes involved in cell proliferation (when activated)
if normal genes promoting cell proliferation are up-regulated thorugh mutation they can predispose the cell to cancer
How may oncogenes be activated? (4 ways)
promotor - 2
multiple copies of the same gene - 3
e.g Philadelphia - 4
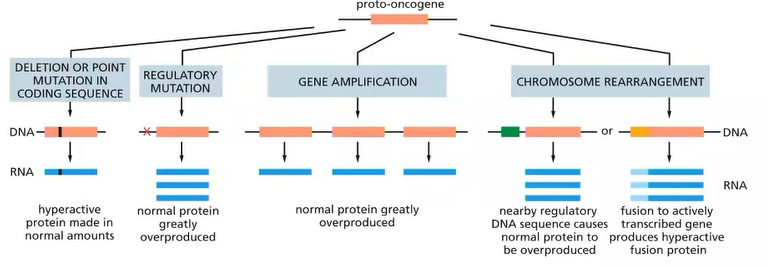
oncogenes function in a dominant fashion, what does this mean?
the gene product of the wildtype allele cannot overcome the effect of the mutated protein
How does RSV cause cancer?
they kidnap cellular proto-oncogenes called Src
retrovirus so integrates into the host genome
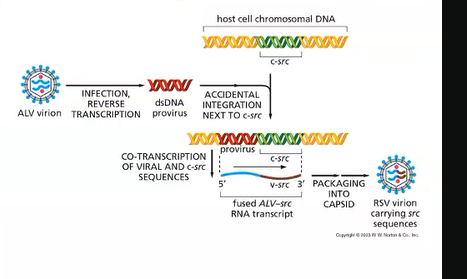
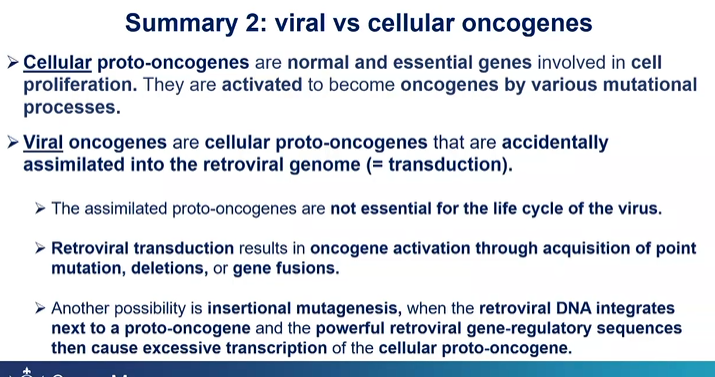
viral vs cellular oncogenes?
another painting of initiator causes development of carcinoma why?
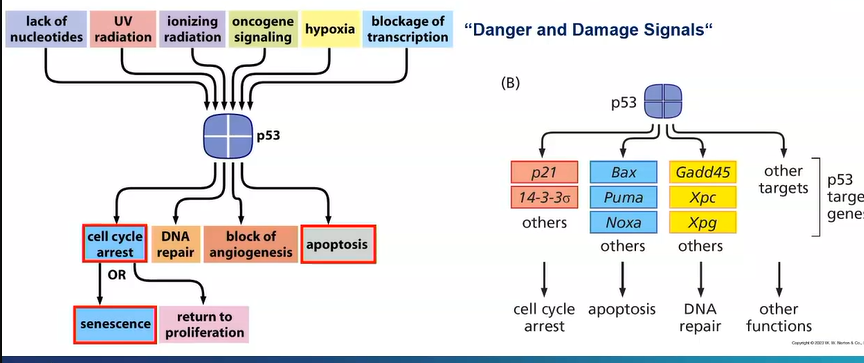
there is a mutation in the Tp53 gene - an essential tumour suppressor gene in most cancers
acts as a TF in response to cellular stress and DNA damage
prevents cells from undergoing cell cycle to allow DNA damage repair
if it can be repaired it allows the cell into cell cycle
if DNA damage is severe, p53 may become quiescent - permanently withdrawn from the cell cycle, it may also instruct apoptosis
Tumour suppressor genes can be grouped into 2 categories?
and give examples for each
caretaker genes - ensure stability of the genome via DNA repair and subsequently when mutated allow more mutations to occur e.g BRCA1 and TP53
gatekeeper genes - directly regulate proliferation by inhibiting cell cycle progression or inducing apoptosis, TP53, RB1
what does tumour suppressor genes being recessive at the cellular level mean?
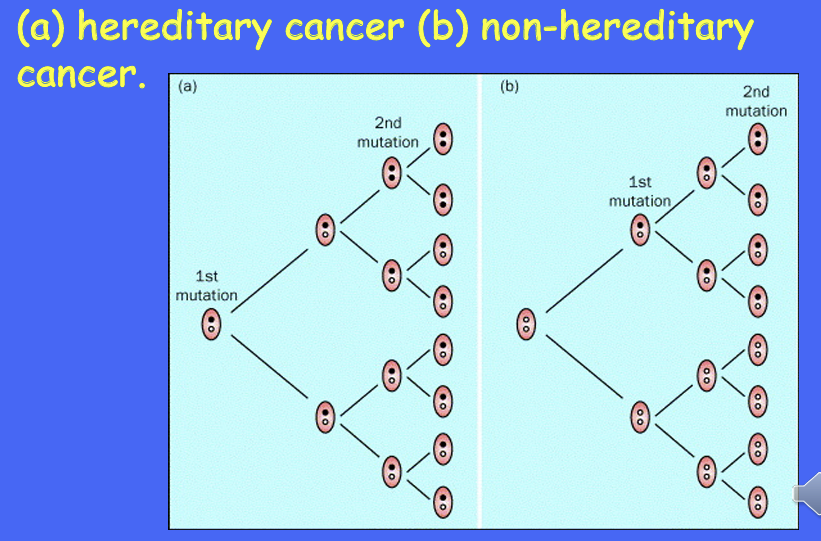
inactivation of both alleles typically found in tumours - 2 hit hypothesis -
real lecture: all cancers have what type of mutations?
somatic mutations
What is polymorphism?
changes in the DNA sequence that occur commonly in the population
what is the weighting of genetic vs environmental influences for retinoblastoma and lung cancer?

give 4 examples of inherited cancers?
retinoblastoma
breast cancer
colon cancer
multiple endocrine tumours
mutations rates throughout life stay constant or vary?
stay constant- although cancer increases markedly with age
the development of cancer requires how many mutaitons?
many
again what is an oncogene?
gain of function of increased function associated with cancers
has potential to cause cancer
in tumour cells, they are often mutated or expressed at high levels
Tumour suppressor gene?
loss of function or reduced function associated with cancer
What is a proto-oncogene?
is a normal gene that can become an oncogene due to mutations or increased expression
code for cell growth and differentiation
often involved in signal transduction and execution of mitogenic signals
the resultant protein is called?
oncoprotein
What is oncogene activation?
the conversion of a proto-oncogene to an oncogene
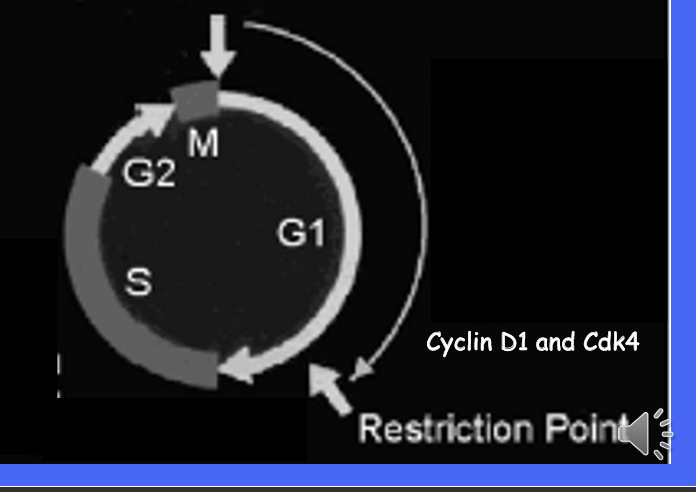
What are 2 proteins important in control of cell cycle G1 to S?
what are kinases and phosphatases?
kinases - add phosphate to amino acids e.g serine, threonine, tyrosine e.g serine kinases
al - remove phosphate from amino acids
protein modifications does what to proteins?
alters conformation and influences protein-protein and protein-nucleic interactions
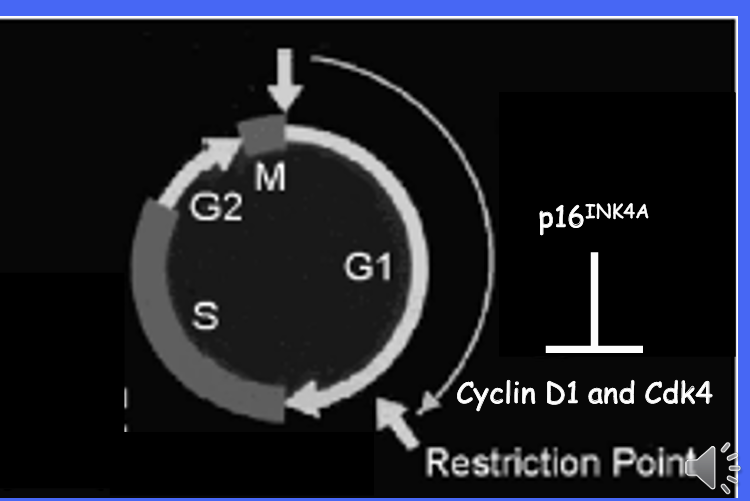
what is the relationship between cyclins and cyclin-dependent kinases?
cyclins and cyclin dependent kinases form complexes
cyclin are regulatory and activate the Cdks
what cancer do cyclins target?
a major target of the cyclin is the retinoblastoma TSG which it phosphorylates at multiple points throughout the cell cycle
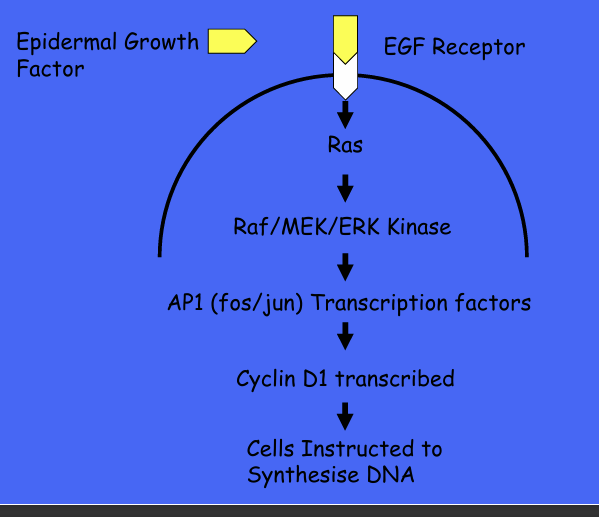
Growth factors and cell signalling cascade?
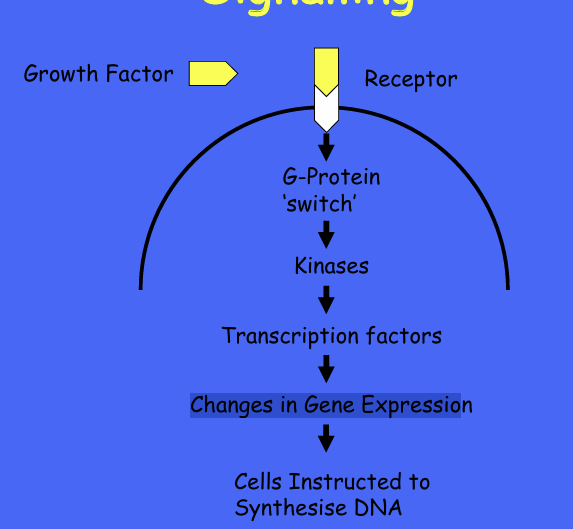
GF and oncogenes and cell signalling -ras-
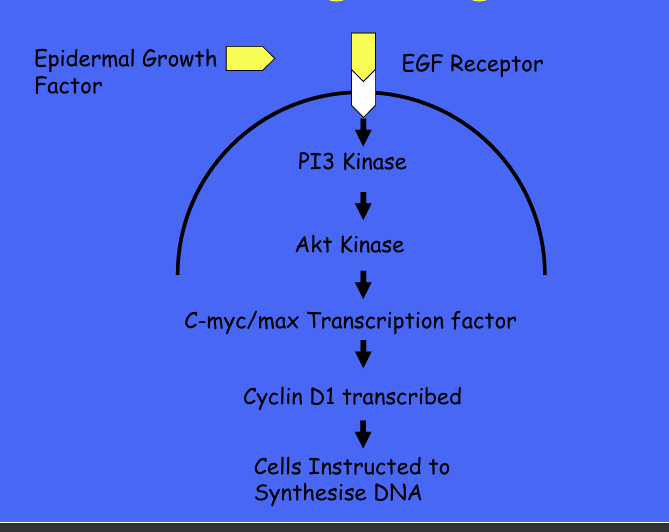
GF and oncogenes and cell signalling - PI3
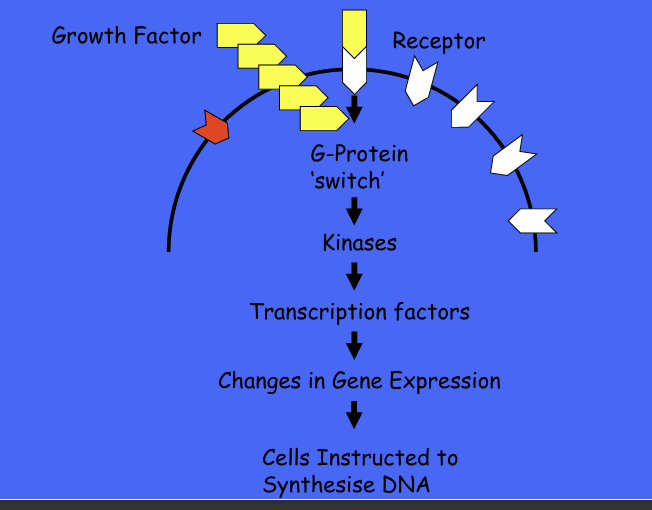
example of overexpressed proto-oncogne and growth factors?
perturbation of GFA SIGNALLING BY OVEREXPRESSION
What is ras?
an example of an oncogene created by mutation of a proto-oncogene
MYC gene implicated in what cancer?
how does is its over-expression caused?
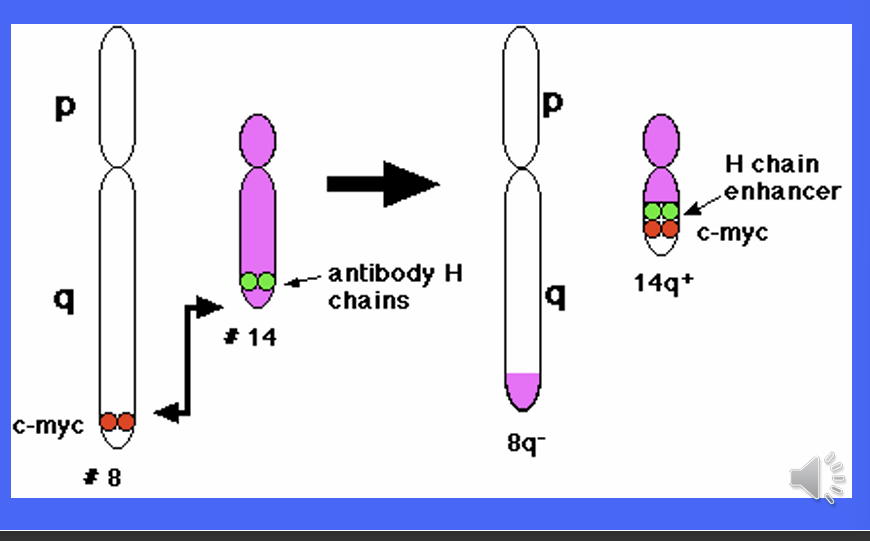
The MYC gene is implicated in Burkitt's Lymphoma, which starts when a chromosomal translocation moves an enhancer sequence within the vicinity of the MYC gene.
• The MYC gene codes for widely used transcription factors.
• When the enhancer sequence is wrongly placed, these transcription factors are produced at much higher rates
What is the Philadelphia chromosome?
The BCR-ABL gene found on the Philadelphia Chromosome, a piece of genetic material seen in Chronic Myelogenous Leukemia caused by the translocation of pieces from chromosomes 9 and 22. •
Bcr-Abl codes for a receptor tyrosine kinase, which is constitutively active, leading to uncontrolled cell proliferation
Name 3 proto-oncogenes activated by increased gene copy number
MYC
Cyclin D1
ERB1 - the epidermal growth factor receptor
TSG loss or reduced function affects which genes and cancers?
retinoblastoma

Knudseo’s 2 hit hypothesis for retinoblastoma can be seen in which cancer?
retinoblastoma
you need a recessive mutation to get the cancer
two types of tumour suppressor genes?

How many mutations needed for a tumour in each

caretaker genes can be inherited in a dominant and recessive form give 2 gene examples and how many further mutations must occur to get a cancer?

give some examples of gatekeeper genes and their functions

What does p16INK4A inhibit?
Give some examples of caretaker genes and their functions?

How may viral oncogenes come about?

What is an important virus that causes cancer?

Summary
