Laboratory Techniques in Pharmaceutical Organic Chemistry
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
122 Terms
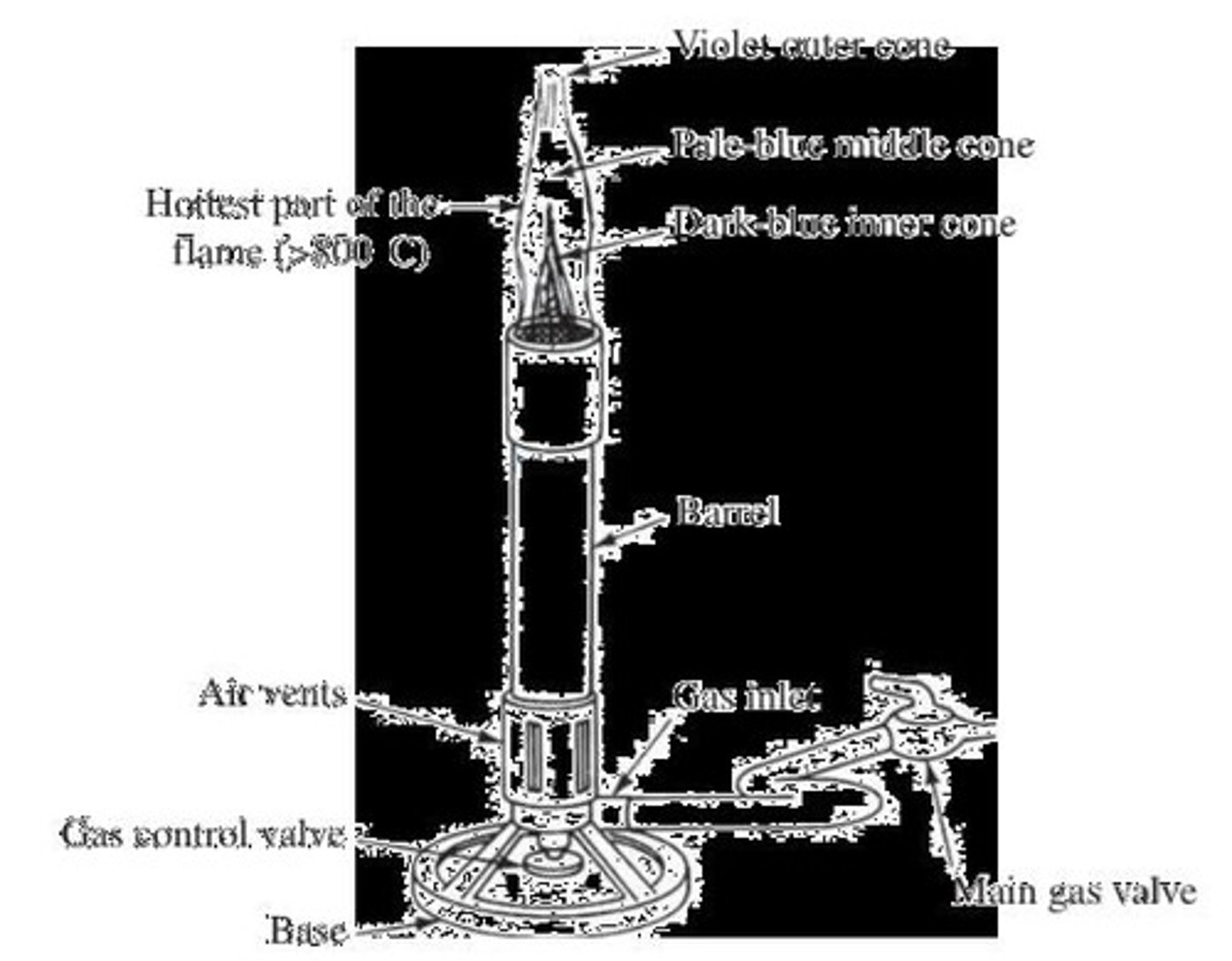
Bunsen burner
A gas burner used for heating substances.
pH measurement
Determining acidity or basicity of a solution.
Litmus paper
Indicator paper for testing pH levels.
Graduated cylinder
Measuring cylinder for precise liquid volumes.
Erlenmeyer flask
Flask with a tapered neck for mixing solutions.
Beaker
Container for mixing and heating liquids.
Triple beam balance
Device for measuring mass with three beams.
Top loading balance
Scale for weighing samples from above.
Analytical balance
High precision scale for measuring small masses.
Hot plate
Heating device for laboratory use.
Wire gauze
Support for containers over a flame.
Thermometer
Instrument for measuring temperature.
Crushed ice
Used for cooling reactions or samples.
Watch glass
Used for holding small amounts of substances.
Dropper
Tool for dispensing small liquid volumes.
Rubber tubing
Flexible tube for gas or liquid transfer.
Match or lighter
Ignition source for the Bunsen burner.
Test tube holder
Device for holding test tubes securely.
Laboratory measurements
Quantitative assessments of physical properties.
Chemical reactions
Processes where substances transform into new products.
Melting point
Temperature at which a solid becomes liquid.
Boiling point
Temperature at which a liquid becomes vapor.
Gas Control Valve
Regulates gas flow to the burner.
Air Vents
Controls air supply for combustion efficiency.
Rubber Tubing
Connects gas inlet to main gas valve.
Main Gas Valve
Primary control for gas supply to burner.
Counterclockwise
Direction to open the gas control valve.
Hiss of Gas
Sound indicating gas flow from burner.
Lighted Match
Used to ignite gas at burner.
Flame Color
Indicates combustion efficiency and air supply.
Test Tube Holder
Safely holds test tube near flame.
Flame Size Adjustment
Controlled by gas control valve position.
Air Vent Adjustment
Modifies flame characteristics by changing airflow.
3-4 Inches High Flame
Desired flame height for optimal burning.
Inner Core of Blue
Indicates complete combustion and proper air mix.
Too Much Air
Can extinguish the flame; close gas valve.
Too Much Gas Pressure
Causes roaring flame; reduce gas flow.
Flashback
Flame at burner base; indicates danger.
Observation Sheet
Records experimental results and observations.
Graduated Cylinder
Measures liquid volume accurately in mL.
Volume Conversion
Convert mL measurement to Liters.
Error Calculation
Determines accuracy of volume measurements.
Percent Error Formula
% Error = (Measured - True) / True x 100.
Graduated Cylinder
Glassware for precise liquid measurement.
Erlenmeyer Flask
Used for mixing and heating liquids.
Beaker
Container for holding and mixing liquids.
Observation Sheet
Document for recording experimental results.
Documentation Sheet
Holds pictures and outputs from experiments.
One Peso Coin
Object used for mass measurement comparison.
Top-Loading Balance
Balance for quick mass measurements.
Analytical Balance
Highly precise balance for small mass measurements.
Weighing Method
Technique to determine mass of objects.
TARE Button
Resets balance to zero before weighing.
Buoyancy Effects
Causes inaccuracies in weight measurements.
Leveling Foot
Adjusts balance to ensure level surface.
Area Calculation
Formula: Area = length x width.
Measurement Conversion
Changing units from cm to mm or m.
Precision Check
Rechecking measurements for accuracy.
Glass Container
Used to hold substances on balance.
Weighing Paper
Protective paper for weighing chemicals.
Chemical Spills
Need immediate cleanup to protect equipment.
Pointer Swing
Indicates balance accuracy during measurement.
Weight Notch
Position where weights are placed on balance.
Measurement Recording
Documenting results in the observation sheet.
Thermometer
Instrument for measuring temperature accurately.
Room Temperature
Typical ambient temperature, around 20-25°C.
Boiling Water
Water at 100°C under standard atmospheric pressure.
Ice Water
Mixture of ice and water at 0°C.
Caution
Warning about handling hazardous materials safely.
Mercury Thermometer
Thermometer using mercury, toxic if broken.
Temperature Conversion
Changing temperature units between °C, °F, and K.
Conversion Formula
Mathematical equations to convert temperature units.
pH Checking
Process to determine acidity or alkalinity of solutions.
Solution A
First unknown solution for pH testing.
Solution B
Second unknown solution for pH testing.
Gas Burner
Device used to produce a flame for heating.
Air Vents
Adjustable openings to control air supply in burner.
Flame Size
Determined by gas flow and air mixture.
Test Tube
Glass container used for holding liquids.
Documentation Sheet
Final compilation of experimental outputs and images.
Watch Glass
Shallow dish used for holding small amounts.
Crushed Ice
Ice broken into small pieces for experiments.
Graduated Cylinder
Used for measuring liquid volume accurately.
Beaker
Used for holding and mixing liquids.
Volume Error Calculation
Difference between measured and actual volume.
Error in Volume Formula
% Error = (Error in volume / Volume by graduated cylinder) x 100.
Triple Beam Balance
Used for measuring mass with three beams.
Top Loading Balance
Used for quick mass measurements.
Length Measurement
Determining the distance between two points.
Area Calculation
Area = length x width.
Room Temperature
Typical indoor temperature, around 20-22°C.
Boiling Water Temperature
Water boils at 100°C at sea level.
Ice Water Temperature
Temperature of ice water is 0°C.
pH Testing
Determining acidity or alkalinity of solutions.
Acidic Solution
Solution with pH less than 7.
Basic Solution
Solution with pH greater than 7.
Volume Measurement Units
Commonly measured in mL and L.
Mass Measurement Units
Commonly measured in g and mg.
Length Measurement Units
Commonly measured in cm, mm, and m.
Temperature Measurement Units
Measured in °C, °F, and K.