Organic Chemistry 2 - Exam 2
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
Conjugated double bonds
C=C double bonds that are separated by one single bond
more stable than isolated double bonds
Isolated double bonds
C=C double bonds that are separated by two or more single bonds
How is diene stability determined?
we examine the heats of hydrogenation
the more stable the compound, the less heat is released (lower heat of hydrogenation)
conjugated double bonds have extra stability —> heat of hydrogenation is less than the sum for the individual double bonds
Stability of carbocations
methyl (least stable) < primary < secondary and allyl < tertiary < substituted allylic (most stable)
1,2- and 1,4- addition to conjugated dienes
electrophilic addition to the double bond produces the most stable intermediate
for conjugated dienes, the intermediate is a resonance-stabilized allylic cation
nucleophile adds to either carbon 2 or 4, both of which have the delocalized positive charge
Which temperatures favor the 1,4 product?
temperatures above 40 degrees Celsius
also called the thermodynamic product
Which temperatures favor the 1,2 product?
temperatures below 0 degrees Celsius
also called the kinetic product
Allylic carbons
the allylic carbon is one directly attached to an sp2 (double-bonded) carbon
allylic cations are stabilized by resonance
Stability of radicals
primary (least stable) < secondary < tertiary < primary allylic
Bromination using NBS
NBS = N-Bromosuccinimide
NBS provides a low, constant concentration of Br2
reacts with the HBr byproduct to produce Br2 and prevent HBr addition across the double bond
bromine has a partial positive charge, making it a good electrophile
during propagation, an allylic radical is formed that is stabilized by resonance
either radical can form the final product
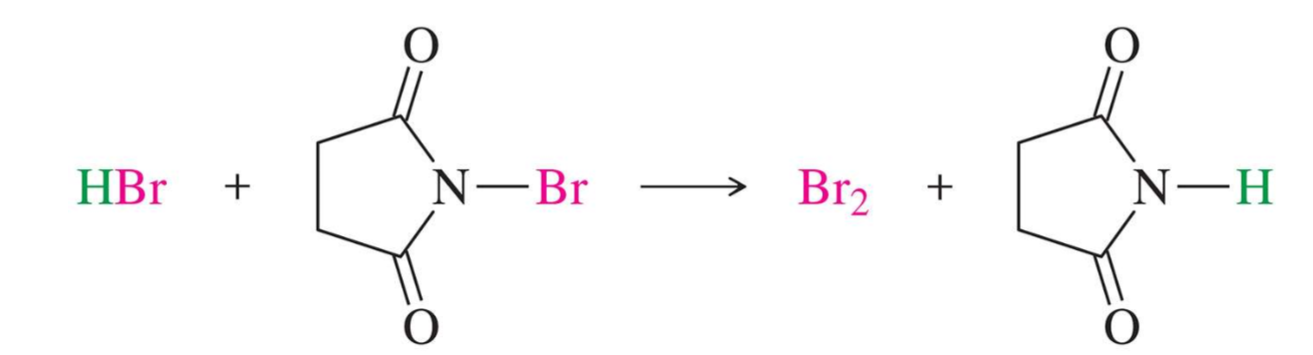
Pericyclic Reactions
conjugated polyenes have the ability to react in these non-ionic, concerted cyclization reactions
these reactions can be easily categorized by the number of pi bonds that are destroyed after a cyclic mechanism
Properties of pericyclic reactions:
Non-ionic —> solvents have no effect on them since there are NO partial charges
Concerted —> all bonds are created and destroyed simultaneously, with NO intermediates
Cyclizations —> mechanisms involve a ring of electrons around a closed loop with cyclic transition states
Reversible —> principle of microscopic reversibility
All can occur either thermally or photochemically
Cycloadditions
pericyclic reactions in which 2 pi-bonds are destroyed after a cyclic mechanism
3 reactant pi-bonds —> 1 product pi-bond
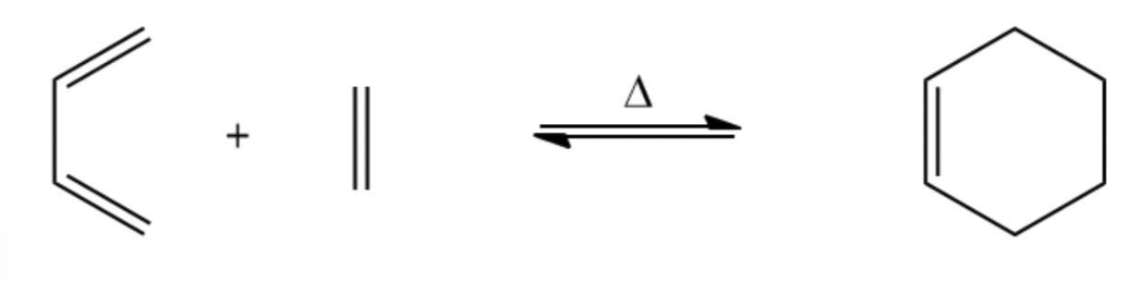
Diels-Alder reaction
reaction is between a 1,3-diene and an electron-deficient/withdrawing alkene (dienophile)
ALWAYS produces a cyclohexene ring
also called a [4+2] cycloaddition because a ring is formed by the interaction of four pi electrons of the alkene with two pi electrons of the alkene or alkyne
Mechanism of the Diels-Alder reaction
one-step, concerted mechanism
a diene reacts with an electron-poor alkene (dienophile) to give cyclohexene or cyclohexadiene rings
a dienophile should contain an electron-withdrawing group
bicyclic bridge products are obtained when s-cis-1,3-diene is cyclic
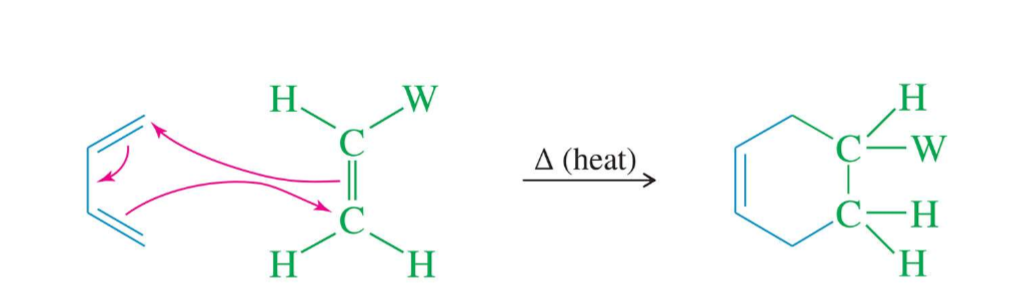
Requirements for Diels-Alder reaction
the diene must be in the s-cis conformation
diene’s C1 and C4 p-orbitals must overlap with the dienophile’s p-orbitals to form new sigma-bonds
both sigma-bonds are on the same face of the diene (syn stereochemistry)
less sterically hindered = faster reaction
stereochemistry of all substituents must be retained
Endo/Exo Stereochemistry
Exo = substituents face towards the bridge (downward)
Endo = substituents face away from the bridge (upward)
when a bridged product is made, substituents must face in the endo direction
Ultraviolet spectroscopy
200- to 400-nm photons excite electrons from a pi-bonding orbital to a pi-antibonding orbital
conjugated dienes have MOs that are closer in energy
a compound that has a longer chain of conjugated double bonds absorbs light at a longer wavelength
nondestructive and exceptionally sensitive
can measure small concentrations of highly conjugated metabolites
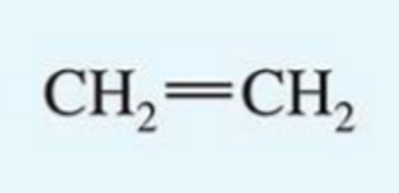
UV absorption maxima of ethylene
171 nm
isolated molecule
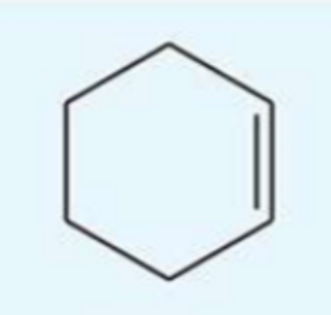
UV absorption maxima of cyclohexane
182 nm
isolated molecule
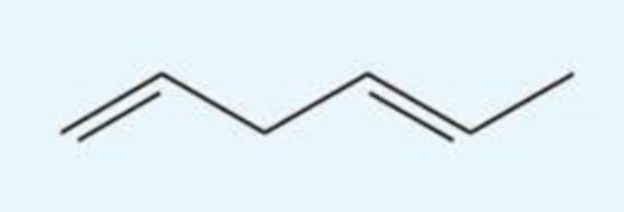
UV absorption maxima of hexa-1,4-diene
180 nm
isolated molecule
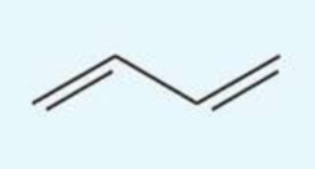
UV absorption maxima of buta-1,3-diene
217 nm
conjugated diene
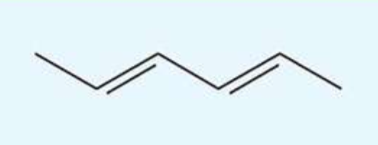
UV absorption maxima of hexa-2,4-diene
227 nm
conjugated diene
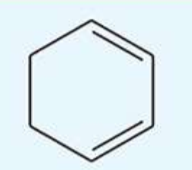
UV absorption maxima of cyclohexa-1,3-diene
256 nm
conjugated diene
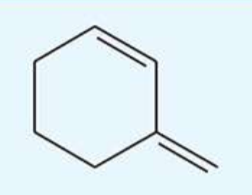
UV absorption maxima of 3-methylenecyclohexene
232 nm
conjugated diene
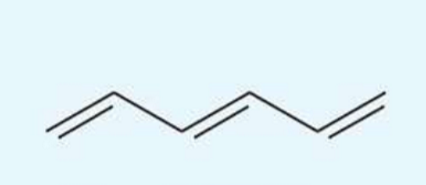
UV absorption maxima of hexa-1,3,5-triene
258 nm
conjugated triene
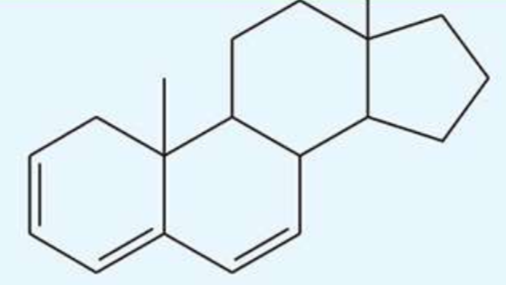
UV absorption maxima of steroid trienes
304 nm
conjugated trienes
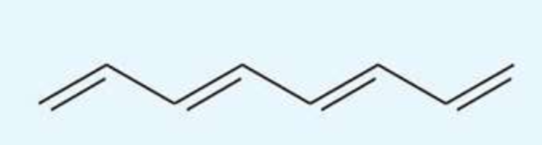
UV absorption maxima of octa-1,3,5,7-tetraene
290 nm
conjugated tetraene
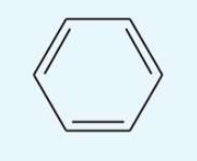
UV absorption maxima of benzene
255 nm
log10E = 2.4
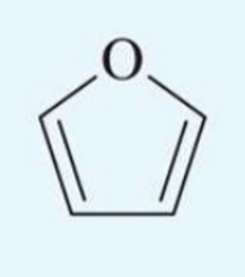
UV absorption maxima of furan
208 nm
log10E = 3.9

UV absorption maxima of pyrrole
324 nm
log10E = 4.47
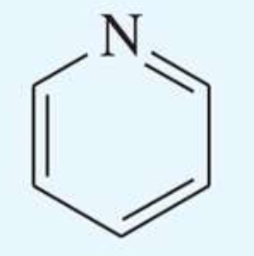
UV absorption maxima of pyridine
256 nm
log10E = 3.1
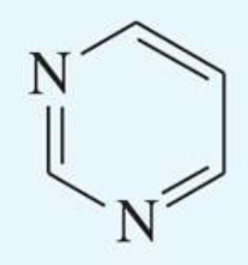
UV absorption maxima of pyrimidine
240 nm
log10E = 3.4
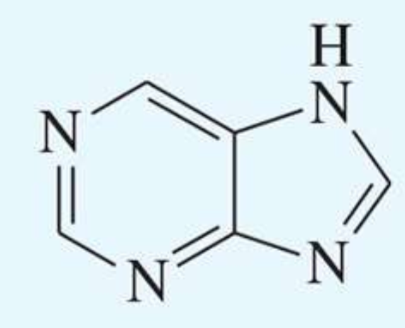
UV absorption maxima of purine
263 nm
log10E = 2.9
Aromatic compounds (arenes)
include benzene and benzene derivatives, like toluene and Xylene
most aromatic compounds are odorless
aromatic rings are a common feature in drugs
rings are largely hydrophobic
Toluene
Phenol
Anisole
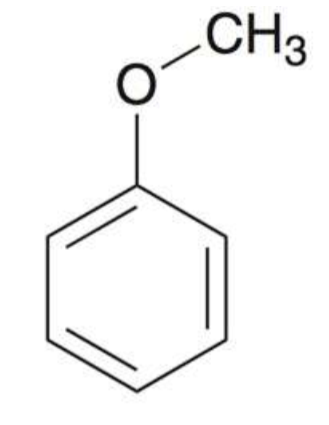
Aniline
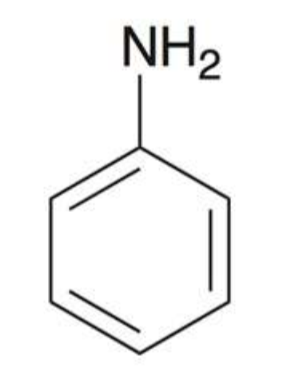
Benzoic acid
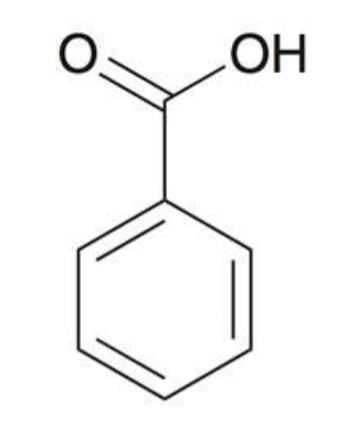
Benzaldehyde
Acetophenone
Styrene
Disubstituted benzene rings
dimethyl benzene derivatives are also called xylenes
three locations: ortho- (1,2), meta- (1,3), and para- (1,4)
Steps for naming benzene derivatives
Identify the parent chain.
Identify and name the substituents.
Number the parent chain and assign a locant to each substituent.
Give the first substituent the lowest number possible.
List the numbered substituents before the parent name in alphabetical order.
Ignore prefixes (except iso-) when ordering alphabetically
Criteria for Aromatic Compounds
A fully conjugated ring with overlapping p orbitals.
Meets Huckel’s rule: an ODD number of electron pairs or [4n + 2] total pi-electrons where n = 0, 1, 2, 3, 4, etc.
Criteria for Antiaromatic Compounds
A fully conjugated ring system with overlapping p orbitals
An EVEN number of electron pairs or 4n total pi-electrons where n = 0, 1, 2, 3, 4, etc.
Nonaromatic compounds
compounds that are NOT fully conjugated rings with overlapping p orbitals
Naphthalene
Anthracene
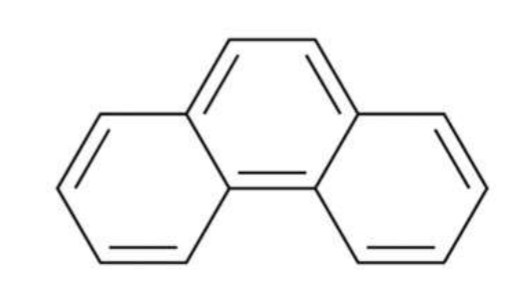
Phenanthrene
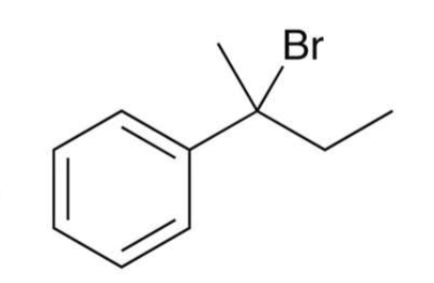
Reactions at the benzylic position
a carbon directly attached to a benzene ring is called a benzylic position
aromatic rings and alkyl groups are not easily oxidized due to their stability
however, benzylic positions are readily oxidized by chromic acid (Na2Cr2O7) or permanganate (KMnO4)
benzylic position needs to have at least 1 proton attached to undergo oxidation
Free radical bromination
benzylic positions are similar to allylic positions —> readily undergo free radical bromination
benzylic bromides are useful synthetic intermediates
readily undergo SN1 substitution
unhindered benzylic bromides can undergo SN2 substitution as well
IR absorption of 3000-3100 cm-1
indicates Csp2 —H stretching
displays one or more signals just above 3000 cm-1
intensity is generally weak or medium
IR absorption of 1700-2000 cm-1
indicates combination bands and overtones
displays a group of very weak signals
IR absorption of 1450-1650 cm-1
indicates stretching of carbon-carbon bonds as well as ring vibrations
displays generally three signals of medium intensity at around 1450, 1500, and 1600 cm-1
IR absorption of 1000-1275 cm-1
indicates C—H bending (in plane)
displays several signals of strong intensity
IR absorption of 690-900 cm-1
indicates C—H bending (out of plane)
displays one or two strong signals
Aromatic protons in 1H NMR
typically appear between 6.5 to 8 ppm
Aromatic integration and splitting of protons
Two of the same substituent: one singlet, 4H
Two different substituents: two doubles, each 2H
Benzene rings in 13C NMR
carbon atoms of benzene typically appear from approximately 100 to 150 ppm
the number of signals can help determine the substitution pattern
Direction groups
all electron-donating groups (activators) are ortho-para directors
all electron-withdrawing groups (deactivators) are meta directors
EXCEPT the halogens
halogens withdraw electrons by induction (deactivating)
but they also donate electrons through resonance (ortho-para directing)
Electrophilic aromatic substitution (EAS) reaction
an aromatic proton is replaced by an electrophile
the benzene ring acts as the nucleophile
the aromaticity of the ring is preserved in the product
Types of EAS reactions
Halogenation
bromination
chlorination
Sulfonation
Nitration
Friedel-Crafts Alkylation
Friedel-Crafts Acylation
Halogenation EAS Reaction
Br2/Cl2 functions as an electrophile during the bromination of an alkene
a Lewis acid catalyst is needed to activate X2 (Br or Cl), making it electrophilic enough to be attacked by the more stable p electrons of an aromatic ring
catalysts: FeX3 or AlX3
Nucleophilic attack —> sigma complex intermediate —> proton transfer
EAS reaction mechanism
Nucleophilic attack
the aromatic ring functions as a nucleophile and attacks the positive or neutral electrophile, forming an intermediate sigma complex (may or may not rearrange)
Proton transfer
in the second step, the sigma complex is deprotonated, restoring aromaticity
Sulfonation
uses SO3 as the electrophile and H2SO4 as the acid catalyst
this reaction is sensitive to reagent concentration —> it is a reversible process
Nitration
uses HNO3 (nitric acid) as the source of the electrophile and H2SO4 as the acid catalyst
believed that a nitronium ion (NO2+) is the active electrophile
a nitro group an be reduced to form an amine using 1) Fe or Zn, HCL and 2) NaOH
Friedel-Crafts Alkylation
use an alkyl halide as the electrophile and AlCl3 as the Lewis acid catalyst
most primary alkyl halides are susceptible to rearrangement in order to become more stable (most substituted spot) —> gives rearranged products
Limitations of Friedel-Crafts Alkylation
The halide leaving group must be attached to an sp3-hybridized carbon in order for the reaction to occur
Polyalkylation often results
Some substituted aromatic rings such as nitrobenzene are too deactivated to react
Friedel-Crafts Acylation
catalyzed by AlCl3 as the Lewis acid
the active electrophile is an acylium ion(R-C+=O) —> resonance stabilized and not subject to rearrangement
AFTER the EAS proton transfer, the product complex must be hydrolyzed (by water) to release the free acylbenzene
polyacylation is generally NOT observed
Clemmensen Reduction
a way to convert acylbenzenes to alkylbenzenes by treatment with aqueous HCl and amalgamated zinc (Zn[Hg] and aq. HCl)
Guidelines for multiple substituents during EAS reactions
Steric hindrance must be considered:
For a monosubstituted ring, the para product typically dominates.
For 1,4-disubstituted rings, substitution will occur at the less sterically hindered site (if more than one site is favored by directing effects)
For 1,3-disubstituted rings, substitution typically does not occur between the existing substituents (you will not have something bond on the second carbon, it would be directed to the other side of the ring)
Synthesis limitations
In most cases, changing the order of the reactions will change the substitution patterns on the ring
Nitration cannot be done on a ring that already contains an amino group —> the NH2 will be oxidized instead
Friedel-Crafts reactions do not work on a ring that is strongly deactivated
Nucleophilic aromatic substitution
a reaction where the benzene is attacked by a nucleophile
occurs under normal temperatures (high temps indicate Elimination-Addition reactions)
Requirements for Nucleophilic Aromatic Substitution (SNAr)
The benzene ring MUST possess a strong electron-withdrawing group (the ring must be electron-poor)
The ring must possess a good leaving group, like a halide.
The leaving group MUST be positioned ortho or para to the withdrawing group.
Nucleophilic Aromatic Substitution Mechanism
Nucleophilic attack
the aromatic ring is attacked by a nucleophile, forming the intermediate Meisenheimer complex
Loss of a leaving group
in the second step, a leaving group is expelled to restore aromaticity
Elimination-Addition Reactions
One halide group —> OH group replaces halides
uses NaOH at high temperature, H3O+
Halide group para to methyl group —> NH2 replaces halide group para or meta to methyl group
uses NaNH2 and NH3(I), H3O+
Birch Reduction
reaction reduces the aromatic ring to a nonconjugated 1,4-cyclohexadiene
reducing agent is sodium or lithium in a mixture of liquid ammonia and alcohol
Mechanism for Elimination-Addition (Benzyne Reaction)
Proton transfer
hydroxide functions as a base and deprotonates the aromatic ring
Loss of a leaving group
a leaving group is ejected, generating a benzyne intermediate
Nucleophilic attack
hydroxide functions as a nucleophile and attacks benzyne
Proton transfer
the resulting anion removes a proton from water to yield the product
Key point of UV-Vis spectrum
As the number of conjugated (consecutive) pi-bonds increases, the energy gap decreases, meaning that light of less energy (longer wavelength) is absorbed
Heterocyclic ring
a carbon-containing ring with one or more carbon atoms replaced by another atom (heteroatom)