Agriculture Biotechnology
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
Benefits of agricultural biotechnology
•Crops with greater resistance to drought,
thus enabling agriculture in drier areas.
•Improved plant resistance to pests and
diseases, reducing the number of
phytosanitary products to be used.
•It allows the creation of varieties that
withstand the use of herbicides, making
weed control easier in large areas of
cultivation.
•It improves the nutritional quality of
agricultural products. For example, it
increases the vitamin content in crops.
How was papaya ringspot virus
(PRSV) engineered?
The papaya ringspot virus
(PRSV) threatened the Hawaiian
papaya industry until genetically
engineered rainbow papayas
resistant to the disease were
created
• This breakthrough saved the US
papaya industry, and research is
ongoing for other crops such as
potatoes, squash, and tomatoes,
to provide resistance to viral
diseases that are challenging to
control.
• Biotech crops have the potential to
increase farming profitability by
improving crop yields, nutrient use
efficiency, resistance to biotic and
abiotic stress, and nutritional profile
Somaclonal variation can occur from ______? (4)
base deletion or substitution,
changes in chromosome number,
chromosome rearrangements,
or changes in epigenetic marks,
like hyper- or hypomethylation.
What types of cells (w examples) are particularly prone to somaclonal variation compared to others?
Undifferentiated cells like protoplasts or calli seem to be particularly prone to
somaclonal variation compared to axillary buds and meristems
What are phenotypic abnormalities?
The observable traits in plants such as height, biomass, leaf shape, color, and so on as ‘phenotype’.
The variations in any of such traits due to tissue culture practices as ‘phenotypic abnormalities’.
4 levels of somaclonal variation
Morphological level
Physiological level
Molecular level
Cytological level
Somaclonal variations: morphological level
1. Morphological Level:
Changes in plant height, leaf shape, color, and size
Altered flower and fruit structure
Variations in root and shoot development
Differences in growth rate and overall plant vigor
Somaclonal variations: physiological level
2. Physiological Level:
Changes in photosynthetic efficiency
reactions of plants to certain stimuli.
Altered response to abiotic stresses (e.g., drought, salinity)
Differences in nutrient uptake and utilization
Variations in flowering time and seed production
Somaclonal variation: Molecular level
Molecular Level:
Detection through DNA markers such as RAPD, SSR, AFLP, and SNPs
Gene expression analysis using RT-PCR or RNA sequencing
Epigenetic changes like DNA methylation and histone modifications
Presence of transposable elements or mutations in specific genes
Somaclonal variations: cytological level
Cytological Level:
Chromosomal abnormalities (e.g., aneuploidy, polyploidy, translocations)
Microscopic examination of mitotic and meiotic cell division
Changes in chromosome number or structure (karyotyping)
Use of fluorescent in situ hybridization (FISH) for chromosomal mapping
Types of somaclonal variations
Gametoclonal variation: variation observed
among the plants regenerated from gametic
cultures.
Androclonal variation: observed among the
plants regenerated from the anther (or) pollen
culture.
Gynoclonal variation: from ovule (or) ovary
culture.
Protoclonal variation: variation observed among
the plants regenerated from protoplast cultures.
Calliclonal variation: variation observed among
the plants regenerated from callus cultures.
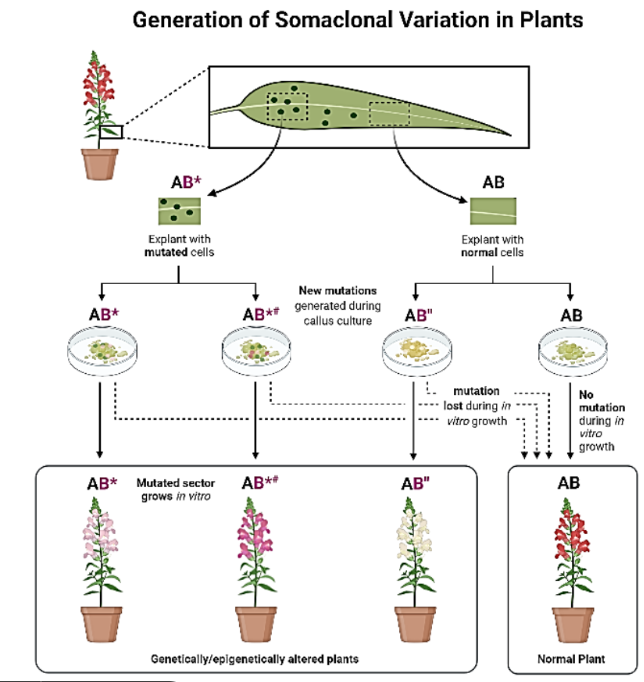
Make a diagram for how somaclonal variations are generated
Explant Selection:
A leaf section is taken as an explant.
It may contain mutated cells (AB*) or normal cells (AB).
Callus Culture and Mutation Generation:
Mutated explants (AB*) may acquire new mutations (AB*#) or lose mutations (AB").
Normal explants (AB) usually remain unchanged, leading to normal plants.
Plant Regeneration:
Plants regenerated from mutated explants (AB*, AB*#, and AB") show genetic and epigenetic variations in traits such as flower color.
Plants from normal explants (AB) remain unchanged.
Factors Causing Oxidative Stress in Tissue Culture
Explant preparation (wounding and sterilization)
Media components (plant growth regulators, salts)
In vitro culture environment (physical state, temperature, light)
What are the consequences of free radical accumulation?
Consequences of Free Radical Accumulation:
DNA methylation changes (hyper/hypo-methylation)
Chromosomal abnormalities (changes in chromosome number)
Chromosomal rearrangements
DNA base mutations (deletion/substitution)
Is somaclonal variation reversible?
Epigenetic Changes (Reversible)
DNA methylation and histone modifications can be reversed by environmental conditions, stress removal, or chemical treatments (e.g., 5-azacytidine for demethylation).
Some tissue-cultured plants may regain normal phenotypes after a few generations.
Genetic Mutations (Irreversible)
DNA base deletions, substitutions, or chromosomal rearrangements are permanent and cannot be reversed naturally.
These mutations are passed on to progeny if they occur in reproductive cells.
Culture Conditions
Some somaclonal variations may disappear when plants are transferred from in vitro to ex vitro conditions, as stress-related epigenetic changes fade.
Selection and Breeding
If undesirable somaclonal variations occur, selective breeding or genetic engineering may be required to restore the original traits.
Advantages of somaclonal variation
Advantages:
Generation of Genetic Diversity
Provides a source of new genetic variation, which is useful for plant breeding and improvement.
Development of Stress-Tolerant Plants
Some somaclonal variants exhibit enhanced resistance to biotic (diseases, pests) and abiotic stresses (drought, salinity, temperature extremes).
Improved Crop Traits
Can lead to higher yield, better quality, early flowering, and altered plant architecture, which are beneficial for agriculture and horticulture.
Rapid Development of New Varieties
Unlike traditional breeding, which takes several generations, somaclonal variation allows for faster selection of improved traits in tissue-cultured plants.
Useful in Micropropagation and Clonal Selection
Helps in selecting superior clones from tissue culture, allowing for large-scale propagation of genetically improved plants.
Overcomes Genetic Barriers
Introduces genetic changes that might not be possible through conventional crossbreeding due to incompatibility issues.
Disadvantages of somaclonal variation
Unpredictability of Variations
Somaclonal variation is random and may produce undesirable changes, making it difficult to control or predict useful traits.
Loss of Genetic Stability
Some variations are unstable and may revert in subsequent generations, leading to inconsistency in plant traits.
Reduction in Plant Vigor and Yield
Some variants may suffer from lower growth rates, reduced fertility, or weaker plants, negatively impacting commercial production.
Risk of Chromosomal Abnormalities
Can introduce harmful mutations, such as chromosomal deletions, translocations, or aneuploidy, leading to reduced plant fitness.
How does Florida beauty have somaclonal variations?
During tissue culture or vegetative propagation, plants are exposed to in vitro stress (e.g., oxidative stress, nutrient variations, and temperature changes).
This stress can trigger mutations or epigenetic modifications that may lead to heat-tolerant variants.
Some somaclonal variants show changes in leaf structure, which can enhance heat and drought tolerance by reducing water loss.
Florida Beauty variants with thicker cuticles or altered stomatal density may have improved tolerance to high temperatures.
Epigenetic changes, such as DNA methylation or histone modifications, can regulate heat shock proteins (HSPs) and other stress-response genes.
Some somaclonal variants may have altered chlorophyll content or enhanced antioxidant systems, which can protect against heat-induced oxidative stress.
Factors that increase the probability of somaclonal variations
Genotype and ploidy level
Composition of growing medium
Duration of callus phase
Proliferation rate
Total time in culture
Factors leading to increase in probability of somaclonal variation: genotype and ploidy level
The Rubus species can have chromosome sets from 2x to 12x genotypes!
Blueberry, potato, and sugarcane are perfect examples of polyploid plants.
The interesting fact is that plant tissue culture can lead to changes in the ploidy level of the plantlets.
A different ploidy level will give plants a different appearance as well as change internal functions.
Unfortunately, ploidy levels are not reversible.
Factors leading to increase in probability of somaclonal variation: composition of growing medium
Some genotypes are more susceptible to abnormalities than others in presence of high plant hormone concentrations.
Growth regulators, such as: 2,4- dichlorophenoxyacetic acid (2,4-D) and 6- benzylaminopurine (BA), have been involved in the induction of somaclonal variation.
Factors leading to increase in probability of somaclonal variation: duration of callus phase
In some cases, the severity of somaclonal variations increases with the age of the callus culture.
The abnormal cells keep on cloning themselves and multiplying as time goes by.
This has been reported in Arabica and Robusta coffee propagation by somatic embryogenesis where prolonged duration of callus phase resulted in variations.
Factors leading to increase in probability of somaclonal variation: Proliferation rate
Tissue culture method thrives on its capability of producing a large number of plants in short periods of time.
In order to have higher proliferation rates, often need to add high amounts of growth hormones in culture media.
This high concentration is one of the major causes of somaclonal variations.
Factors that lead to increase in probability of somaclonal variations: total time in culture
In plant tissue culture it is better to minimize the amount of time that a culture is maintained in vitro. Long-term cultures can be an excellent source of variants.
Why is agitation required in cell suspension cultures?
It exerts a mild pressure on the tissue and breaks it into smaller cells/pieces
It helps maintain uniform distribution and movement of cells in a medium
How are Camrbium meristematic cells isolated?
Vascular Cambium of woody plants
Enzymatic digestion (cellulase, pectinase) separates CMCs from other tissues
They are placed in MS medium
Supplemented with plant growth regulators (PGRs) like:
Auxins (e.g., 2,4-D, NAA) – for cell division.
Cytokinins (e.g., BAP, Kinetin) – for differentiation control.
Sucrose (2-3%) as an energy source.
pH adjusted to 5.5 - 6.0 for optimal growth.
What are dedifferentiation cell suspension cultures
Dedifferentiation is the process by which specialized (differentiated) cells revert to a less specialized, meristematic state, regaining the ability to divide and form new cell types. This phenomenon is crucial in plant tissue culture, wound healing, and regenerative growth.
How are dedifferentiated cell suspension cultures created?
Explant (e.g., leaf, stem, root) is placed on solid Murashige and Skoog (MS) medium with:
Auxin (e.g., 2,4-D, NAA) – promotes cell division.
Cytokinin (e.g., BAP, kinetin) – regulates shoot induction (optional).
Callus formation occurs within 2-4 weeks.
Callus is transferred to liquid MS medium with the same hormonal balance.
Four characteristics of adventitious root cultures
Originate from non-root tissues under the influence of plant hormones.
Have a high growth rate compared to naturally occurring roots.
Often more efficient in nutrient absorption and metabolite production.
Can be induced under in vitro conditions using appropriate growth regulators.
Creating adventitious root cultures
Explant Selection – Leaves, stems, hypocotyls, or callus are commonly used.
Culture Medium – Typically Murashige and Skoog (MS) or Gamborg’s B5 medium.
Growth Regulators:
Auxins (e.g., IBA, NAA, IAA) – Essential for root induction.
Low cytokinin concentration (or none) to prevent shoot formation.
Incubation – Dark conditions or low light at 22-25°C.
Diagram of plant layers
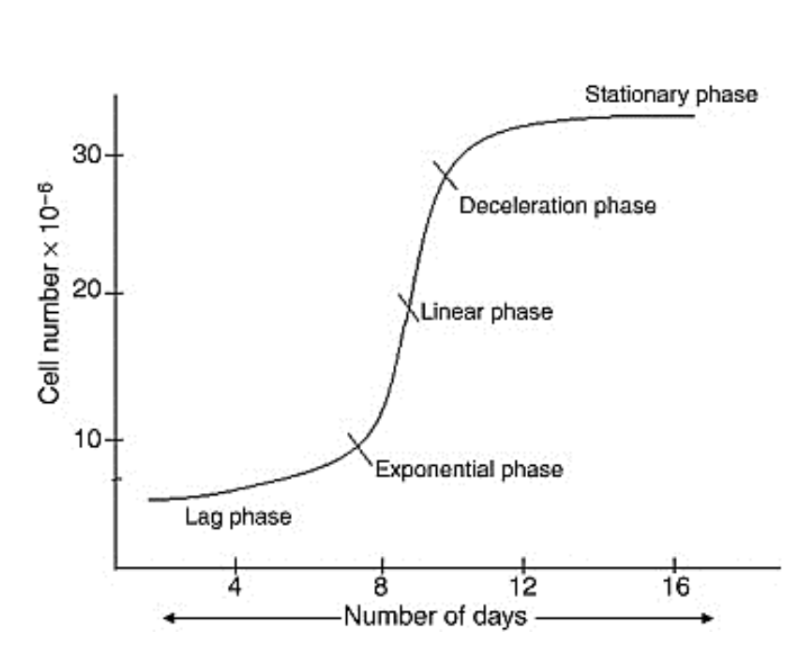
What is a batch culture? Key characteristics
Closed system – No addition of fresh medium during growth.
Growth phases – Follows a characteristic growth curve (lag, exponential, stationary, decline).
Limited by nutrients and waste accumulation – Culture duration depends on the depletion of essential nutrients.
Easy setup and low maintenance – Suitable for short-term experiments and commercial applications.
Limitations of batch cultures
that the cells grow up to a certain point and then the cell growth becomes stationary.
the number of cells and size of cells remains constant.
Cells reach the stationary phase either because
of exhaustion of some growth factors or
accumulation of toxic metabolites in the culture
medium. This phase can be avoided by providing fresh media at a faster rate
What are continuous cultures?
Continuous culture is an open system in which plant cells, tissues, or microorganisms are grown in a bioreactor or liquid medium with constant renewal of nutrients and removal of waste. Unlike batch culture, where nutrients are limited, continuous culture maintains steady-state growth, making it ideal for large-scale biomass production, secondary metabolite synthesis, and plant cell studies.
4 characteristics of continuous cultures
Open system – Fresh medium is continuously supplied while spent medium is removed.
Steady-state growth – Cells maintain constant biomass and metabolic activity.
No lag or decline phases – Growth remains stable, avoiding nutrient depletion.
Higher productivity – More efficient than batch culture for long-term production.
Describe the two types of continuous cultures
1. Chemostat Culture
A chemostat controls the growth rate by limiting a key nutrient in the culture medium. The flow rate of fresh medium determines the rate of cell division.
Key Features:
The growth rate is controlled by the concentration of a limiting nutrient (e.g., nitrogen, phosphate, or carbon source).
Fresh medium is continuously supplied at a fixed rate, and spent medium (with waste) is removed.
The dilution rate (D) (rate of medium flow per unit culture volume) controls cell density.
Cells remain in the exponential phase as long as conditions are stable.
2. Turbidostat Culture
A turbidostat maintains a constant cell density by adjusting the flow of fresh medium based on optical density (turbidity) measurements.
Key Features:
Growth is not limited by nutrients but instead controlled by real-time monitoring of cell concentration.
Fresh medium is added whenever turbidity exceeds a set threshold, keeping cell density constant.
The dilution rate adjusts automatically based on cell growth.
Examples of chemostate and turbidostat cultures
1. Chemostat Culture
Production of shikonin (a red pigment) in Purple Gromwell in cell cultures
2. Turbidostat Culture
Cultivation of Coffea arabica cells for caffeine production.
Advantages of chemostat culture
✅ Precise control of growth rate – Useful for studying metabolic activity.
✅ Efficient nutrient use – Reduces waste compared to batch culture.
✅ Ideal for secondary metabolite production – Optimized conditions improve yields.
Advantages of turbidostat culture
✅ Higher growth rates – Cells grow as fast as possible.
✅ Ideal for fast-growing plant cell lines – Prevents overcrowding and nutrient depletion.
✅ Better suited for large-scale bioprocessing – Used in commercial metabolite production.
How do you create a continuous culture?
Step 1: Setup and Medium Preparation
Use a bioreactor or flask with an inlet and outlet system.
Murashige and Skoog (MS) or B5 medium with sucrose (2-3%) is used.
Growth regulators (e.g., auxins, cytokinins) added based on cell type.
Step 2: Inoculation and Growth
Cell suspension cultures are inoculated into the liquid medium.
Agitation (80-150 rpm) and aeration ensure uniform distribution.
Temperature (22-25°C) and light conditions optimized for plant species.
Step 3: Continuous Medium Flow
Fresh medium is pumped in at a controlled rate.
Spent medium with waste and dead cells is removed.
Step 4: Monitoring and Harvesting
Cell density and metabolite production are measured regularly.
Cells or metabolites are harvested at optimal productivity levels.
Name 4 types of methods to evaluate the viability of cells
Phase-contrast microscopy
Tetrazolium salt reduction
Fluorescein diacetate (FDA) method
Evan's blue staining
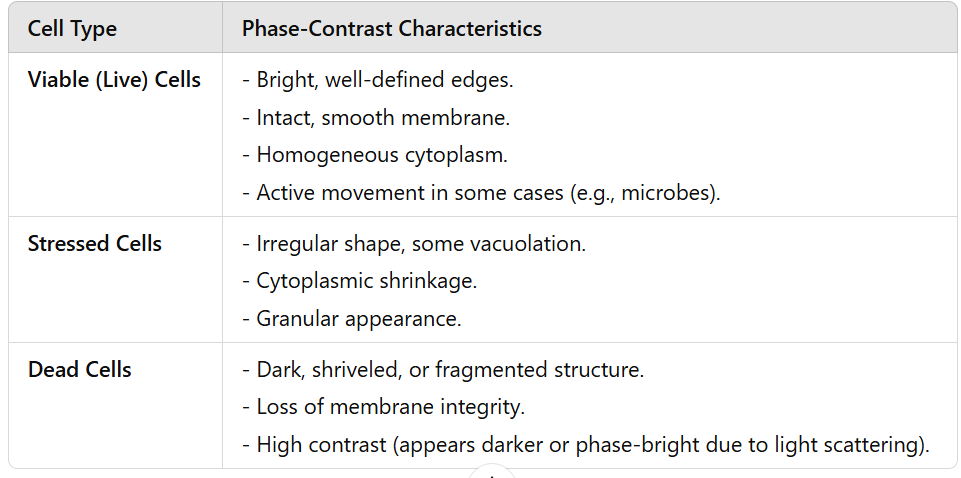
Phase-contrast microscopy principle
Light passing through a specimen is diffracted (slowed down) based on the refractive index of different structures.
A phase plate in the microscope enhances these small phase differences.
The result is a high-contrast image where denser structures appear darker against a lighter background.
phase contrast microscopy: characteristics of live, stressed, and dead cells
Principle of Tetrazolium Salt Reduction
Tetrazolium salts (e.g., MTT, TTC, XTT, INT) are colorless in their oxidized form.
Live cells contain active dehydrogenase enzymes that reduce tetrazolium salts into insoluble or soluble formazan, which has a distinct color (e.g., purple, red, or blue).
Dead or non-viable cells do not reduce tetrazolium salts due to loss of metabolic activity.
Name 2 common tetrazolium salts used

Principle of the FDA Assay to assess cell viability
FDA is a non-fluorescent, lipophilic dye that passively enters live cells.
Inside viable cells, intracellular esterases hydrolyze FDA, releasing fluorescent fluorescein.
Dead or non-viable cells lack esterase activity, so they do not produce fluorescence.
Fluorescence intensity correlates with cell viability and metabolic activity.

Principle of Evan's blue staining
Evans Blue is an anionic dye that cannot cross intact cell membranes.
Live (viable) cells exclude the dye and remain unstained.
Dead or damaged cells have compromised membranes, allowing the dye to enter and bind to cytoplasmic components, resulting in blue staining.
Strategy to achieve high-yield protein
Molecular Approaches
Gene of Interest: The process begins with the selection of a target gene encoding the desired protein.
Expression Vector Optimization: This involves inserting the gene into an expression vector to enhance production efficiency.
Techniques to Increase Yield:
Enhancing gene transcription for higher mRNA levels.
Improving translation efficiency for better protein synthesis.
Minimizing post-translational degradation to prevent protein loss.
Using protein fusion and Hyp-Glyco technology for stability and improved expression.
Cell Culture Approaches
The gene is introduced into a plant cell "factory", where plant cells act as biofactories for protein production.
Strategies to enhance cell culture efficiency include:
Optimizing culture medium for better growth and yield.
Cell immobilization to maintain cell viability and productivity.
In-situ protein removal to prevent degradation and facilitate easy purification.
Bioreactor Engineering
Scaling up the process using bioreactors for mass production of proteins.
Enhancements include:
Optimizing bioreactor design and operation to improve efficiency.
Advanced bioreactor culture strategies, such as controlled environmental conditions for maximum protein synthesis.
Adoption of disposable bioreactors to reduce contamination risks and streamline production.
Downstream Processing
After production, proteins must be purified for commercial or research use.
Purification strategies include:
Advanced chromatographic techniques to separate proteins based on size, charge, or affinity.
Affinity chromatography for high-purity protein isolation.
Membrane separation approaches to remove impurities efficiently.
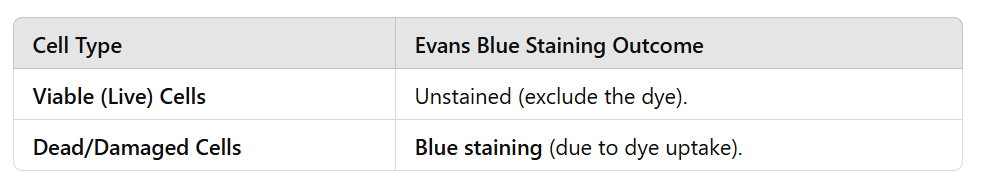
Explain the establishment of cell suspension cultures of Ocimum basilicum L. (basil) for the enhanced production of active pharmaceutical ingredients (APIs).
Diagram
Initiation of Cultures
Seeds of Ocimum basilicum are germinated under controlled conditions.
Callus cultures are developed from plant tissue (likely leaf, stem, or root explants) on an appropriate growth medium.
Callus is transferred into liquid media to establish cell suspension cultures, which allow rapid growth and metabolite production.
Elicitor Treatments
Chemical elicitors such as:
CdCl₂ (Cadmium chloride)
AgNO₃ (Silver nitrate)
Yeast extract
These are added to enhance secondary metabolite production, which includes flavonoids, phenolic compounds, and essential oils.
Extraction and Analysis
Parameters measured include:
Cell growth (cell number, dry weight, viability).
Total phenolic and flavonoid content (antioxidant properties).
Radical scavenging activity (DPPH assay for antioxidant potential).
Specific bioactive compounds such as:
Cichoric acid
Rosmarinic acid
Rutin
Isoquercetin
Essential oils (Estragole, Linalool)
Analytical Techniques for Metabolite Detection
Spectrophotometry: Used to measure total phenolics, flavonoids, and antioxidant activity.
RP-HPLC (Reverse Phase High-Performance Liquid Chromatography): Separates and quantifies bioactive compounds like rosmarinic acid, rutin, and isoquercetin.
HS-SPME-GC/MS (Headspace Solid-Phase Microextraction Gas Chromatography/Mass Spectrometry): Identifies and quantifies volatile essential oils such as linalool and estragole.
Strategies to overcome plant-modification limits
i. Gene and Construct Consideration
ii. Modulation of Chaperone Expression
iii. Limiting in Planta Proteolytic Degradation
iv. Modulation of Endogenous Oxidase Activity
v. Glycosylation of Plant-Produced Vaccine
vi. Tyrosine O-Sulfation of Plant-Produced Vaccines
Strategies to overcome plant-modification limits: Gene and Construct Consideration
Optimizing codon usage to match plant preferences.
Incorporating regulatory sequences (e.g., promoters, enhancers) to boost expression.
Adding signal peptides for correct cellular targeting.
Strategies to overcome plant-modification limits: Modulation of Chaperone Expression
Overexpression of molecular chaperones (like BiP, HSPs) to assist in proper protein folding and assembly.
Helps stabilize complex or multimeric proteins that would otherwise misfold.
Strategies to overcome plant-modification limits: Limiting In Planta Proteolytic Degradation
Co-expressing protease inhibitors or modifying expression conditions to reduce degradation.
Targeting expression to compartments like the ER or chloroplasts for protection from proteases.
Strategies to overcome plant-modification limits: Modulation of Endogenous Oxidase Activity
Regulating reactive oxygen species (ROS)-related enzymes to minimize oxidative damage to recombinant proteins.
Helps preserve protein structure and function.
Strategies to overcome plant-modification limits: Glycosylation of Plant-Produced Vaccines
Engineering glycosylation pathways to produce human-like N-glycans.
Prevents immunogenic plant-specific glycoforms and enhances protein efficacy.
Strategies to overcome plant-modification limits: Tyrosine O-Sulfation of Plant-Produced Vaccines
Introducing tyrosylprotein sulfotransferases to perform tyrosine sulfation—a PTM important for certain protein–protein interactions.
Can improve vaccine antigenicity and mimic native human proteins more accurately.
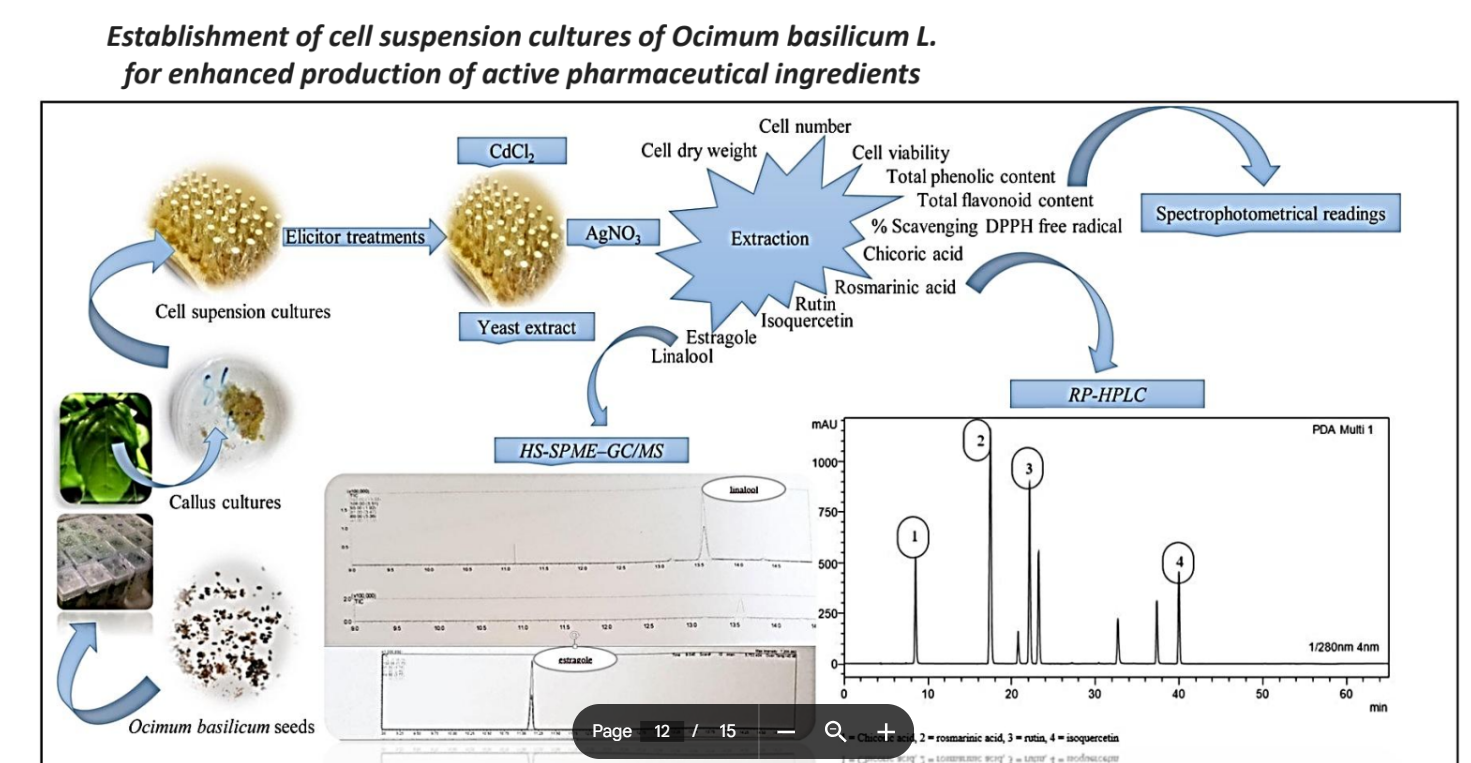
Advantages of plant molecular farming
side stream products
systemic proteins
secondary products
sustainibilility
scalability
speed
safety
simplicity
Challenges
Plants often struggle to properly fold complex proteins, particularly those requiring precise 3D structures
plants lack certain molecular chaperones, which are critical for assisting protein folding.
Plants are inherently equipped with a strong proteolytic system to degrade unwanted proteins
There is a potential for contamination by plant viruses or other pathogens
Diagram for side stream processing
Why is chloroplast engineering better than nuclear engineering?
Higher Expression Levels: Chloroplasts have a high number of copies of their genome within a single cell (thousands of chloroplasts per cell), which can result in much higher protein expression levels
No Risk of Transgene Flow: Chloroplasts are maternally inherited, meaning they are passed down only through the mother plant, reducing the risk of transgene flow to wild relatives.
Reduced Silencing of Transgenes: In plants, nuclear genes can sometimes be silenced due to DNA methylation or histone modification, especially if foreign genes are integrated in a way that activates plant defense mechanisms.
What can enginerred chloroplasts be used for?
therapeutic proteins
biofuel
phytoremediation
industrial enzymes
improved crop production
Steps of chloroplast engineering + diagram
Genes of interest (X, Y, Z) are inserted into chloroplast transformation vectors along with a promoter (P), ribosome binding site (RBS), and terminator (T)
These vectors can carry single or multiple genes, allowing for complex metabolic engineering.
A selectable marker gene (e.g., for antibiotic resistance) is used to identify successful transformants.
Gene delivery is performed (often via biolistic particle delivery or "gene gun").
Regeneration of transformed plants happens under selective pressure:
With betaine aldehyde: higher transformation efficiency.
With antibiotics: lower transformation efficiency.
Successfully transformed plants are regenerated from selected cells.
Initially, the plant cell becomes heteroplasmic, meaning it contains both transformed and untransformed chloroplasts.
After repeated selection rounds, a homoplasmic state is achieved, where all chloroplasts in a cell carry the transgene.
Applications of chloroplast engineering (multiple gene)
Bt operon
PHB operon
Mer operon
Vitamin A genes
plantibodies
monoclonals

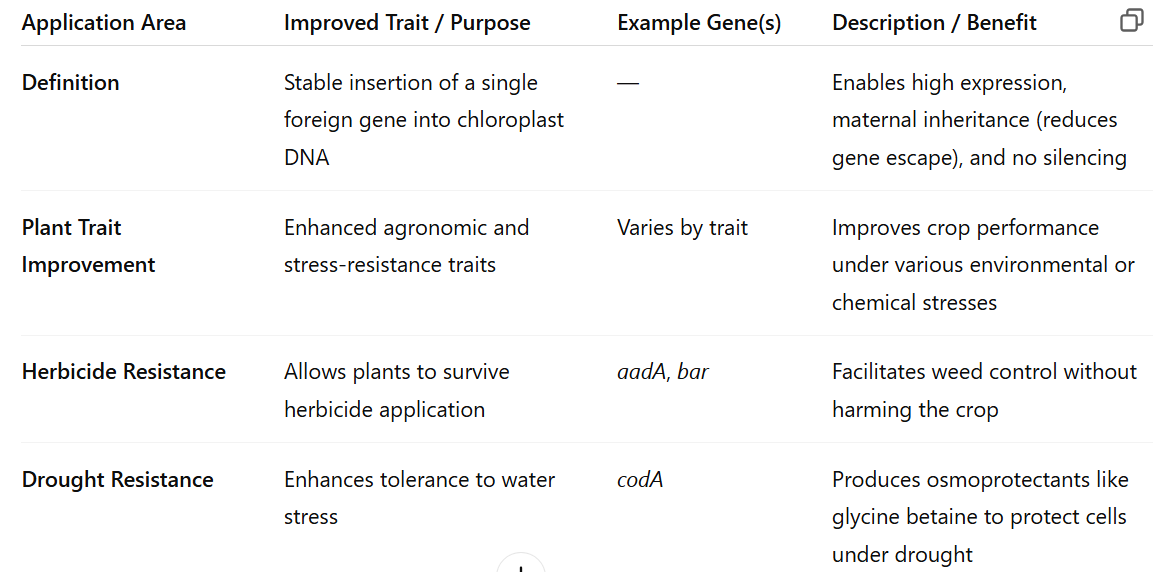
Applications of chloroplast engineering (single gene)
plant traits
herbicide resistance
drought resistance
biopharmaceuticals
edible vaccines
Why is a selectable marker gene used in chloroplast engineering?
The selectable marker gene gives the transformed cell a survival advantage—for example, resistance to an antibiotic or a herbicide.
After the transformation process (such as using a gene gun), the plant cells are placed on a medium that contains the selective agent (e.g., an antibiotic like spectinomycin or kanamycin).
Only those cells that have successfully taken up the foreign DNA (including the marker gene) will survive and grow.
Non-transformed cells, which lack the marker gene, will die in this selective medium.
Cargo delivery in plants using RNA viruses
RNA Virus Selection
Plant RNA viruses such as Tobacco mosaic virus (TMV), Potato virus X (PVX), Barley stripe mosaic virus (BSMV), and Foxtail mosaic virus (FoMV) are commonly used. These viruses have:Well-understood genomes
Broad host ranges
Ability to systemically spread in plants
Engineering the Viral Genome
Scientists insert genes of interest (GOIs) or cargo molecules (like small RNAs, CRISPR components, or proteins) into the viral genome.
The cargo is expressed in infected plant cells as the virus replicates.
Inoculation into Plants
The engineered virus is delivered to plants using mechanical inoculation, Agrobacterium-mediated delivery, or rub inoculation.
The virus spreads through the vascular system, delivering the cargo systemically.
Transient Expression
This system is typically used for transient expression (temporary, non-heritable), making it suitable for rapid testing or temporary trait introduction without genetic modification of the plant genome.
why is viral vector mediated transgenesis faster than traditional transgenesis?
No Genome Integration:
Viral vectors deliver genes without integrating into the plant genome, avoiding the slow and inefficient process of stable transformation.No Tissue Culture Needed:
Traditional methods require regeneration from transformed cells, which takes weeks. Viruses infect whole plants directly.Rapid Systemic Spread:
Viruses naturally move through the plant, allowing fast, widespread gene expression in days.
Modes of introduction of viral vectors for protein expression in whole plants
Mechanical Inoculation (Rub-Inoculation):
Rub viral vector solution onto plant leaves, commonly used for viruses like TMV and PVX.Agrobacterium-Mediated Delivery (Agroinfiltration):
Use Agrobacterium to deliver viral vectors into plant cells, often used in Nicotiana benthamiana.Biolistic Delivery (Gene Gun):
Shoot gold or tungsten particles coated with viral DNA into plant tissue, useful for monocots.Direct RNA Transcripts:
Apply in vitro–transcribed viral RNA directly to plant leaves or inject.Insect Vector Transmission:
Use insects like aphids to naturally transmit viral vectors to plants.
Difference between agrobacterium vs viral vector in cell to cell spreading
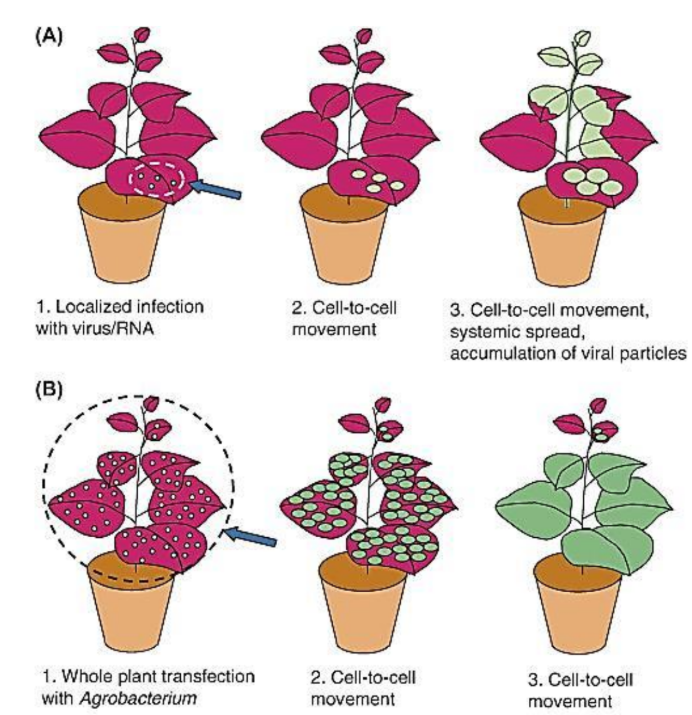
Compare original viral vectors with each generation
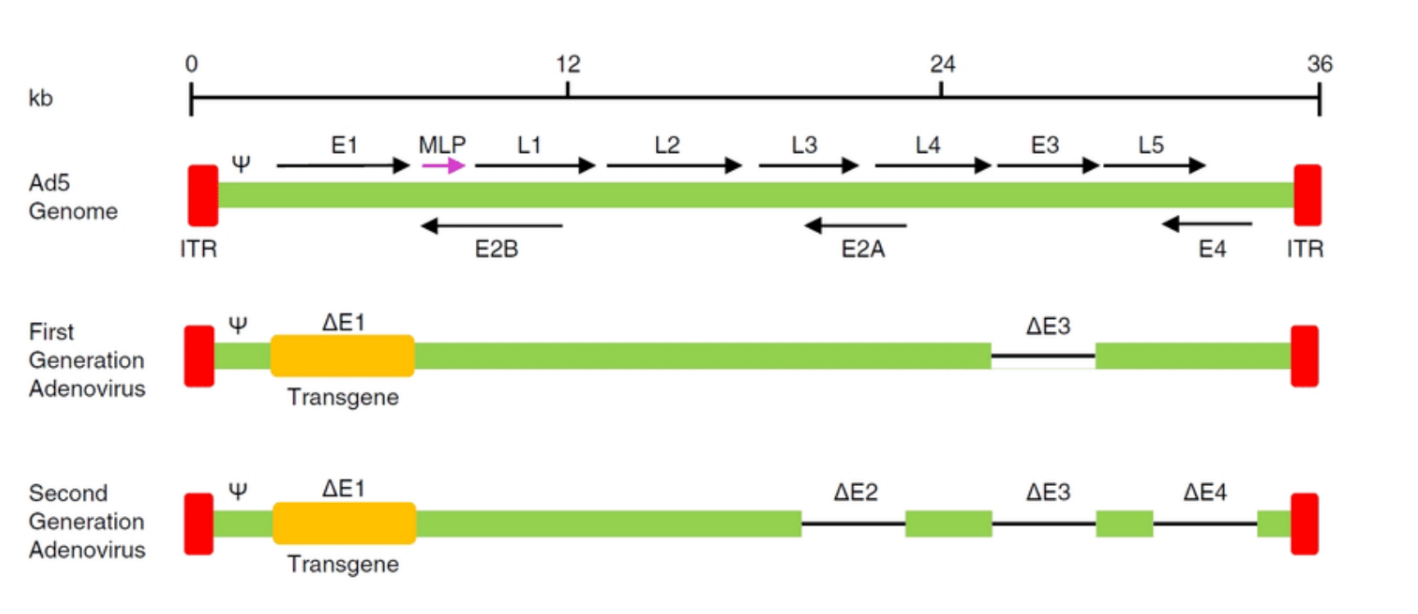
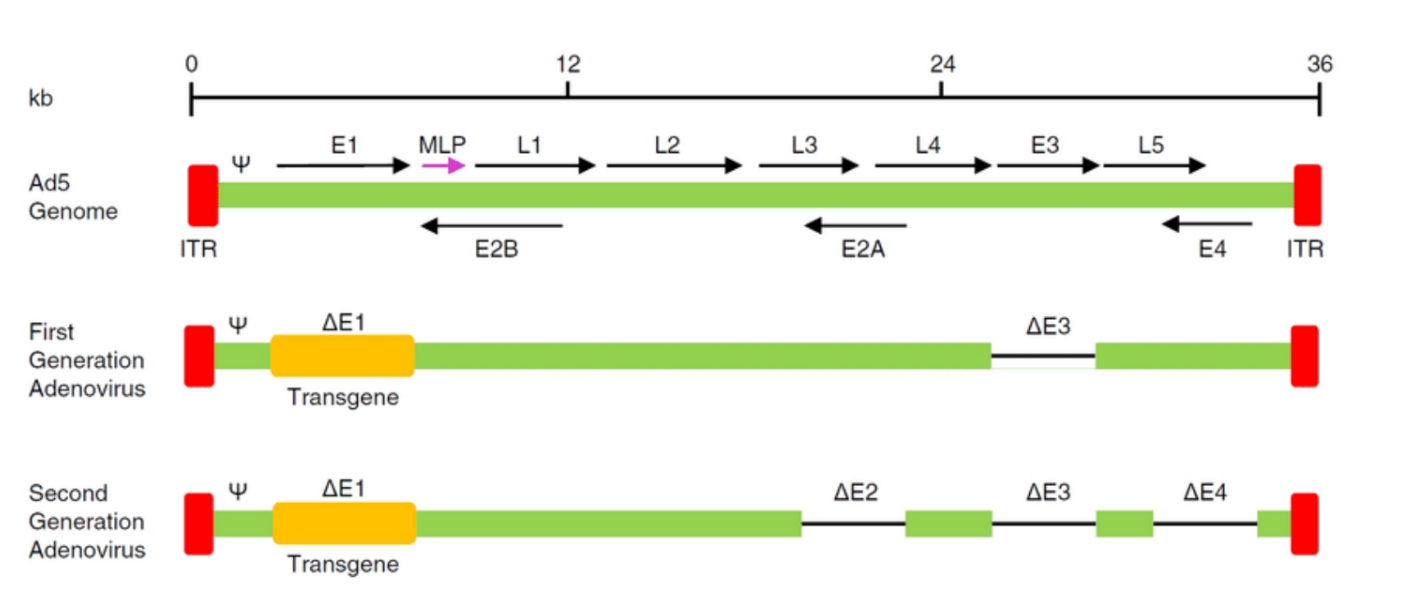
What are MLPS?
Function: A shortened version of the Long Terminal Repeat (LTR) promoter, commonly used in retroviral or lentiviral vectors. It provides the necessary elements for initiating transcription of the inserted gene, but it's minimal to avoid unwanted viral gene expression.
Role in Vectors: Ensures efficient expression of the transgene while minimizing unwanted viral gene expression.
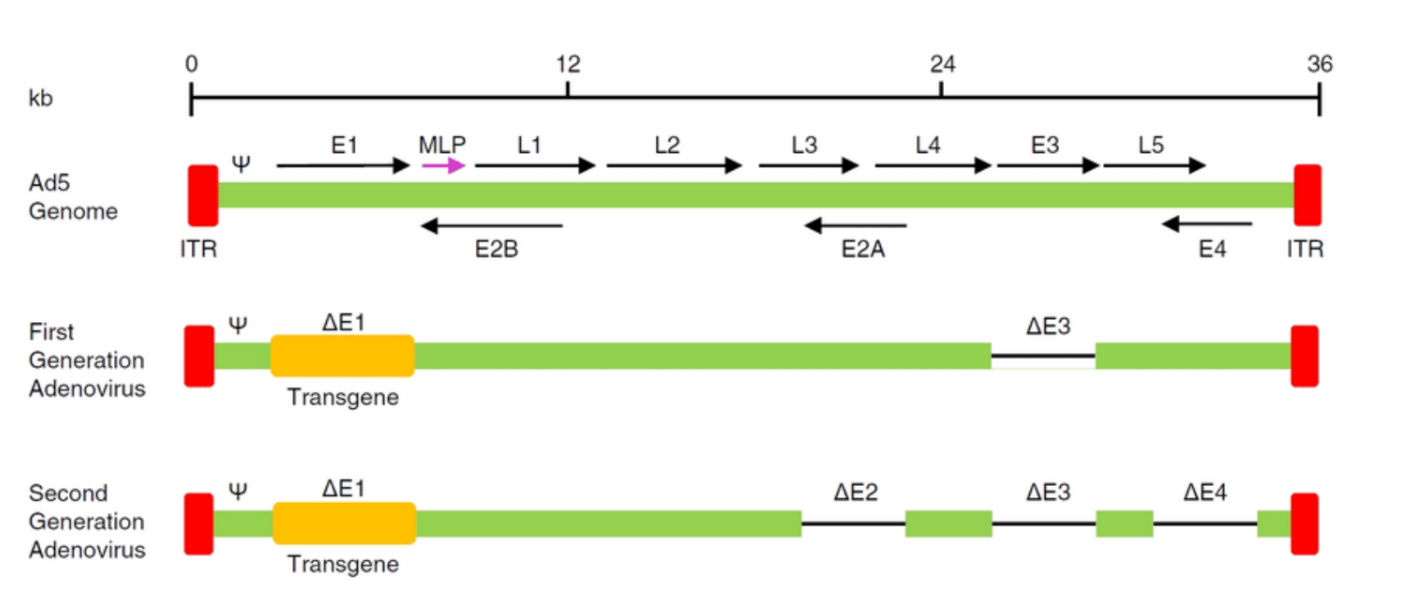
What are E1, E2, E5?
Function: Essential for viral replication and transcription in adenoviral vectors. It encodes proteins required for the early phase of virus replication and for initiating the transcription of viral genes.
In Vectors: Often deleted in recombinant vectors to prevent virus replication in the host, leaving only the ability to deliver the desired gene.
E2:
Function: Encodes proteins involved in the replication of the viral genome (like the DNA polymerase).
In Vectors: Like E1, the E2 region is often deleted in vectors, leaving it dependent on helper systems or cells for replication.
E5:
Function: Encodes a protein involved in regulating the host cell's immune response and influencing cell growth.
In Vectors: Often manipulated in vectors to reduce immune responses or to enhance the stability of gene expression.
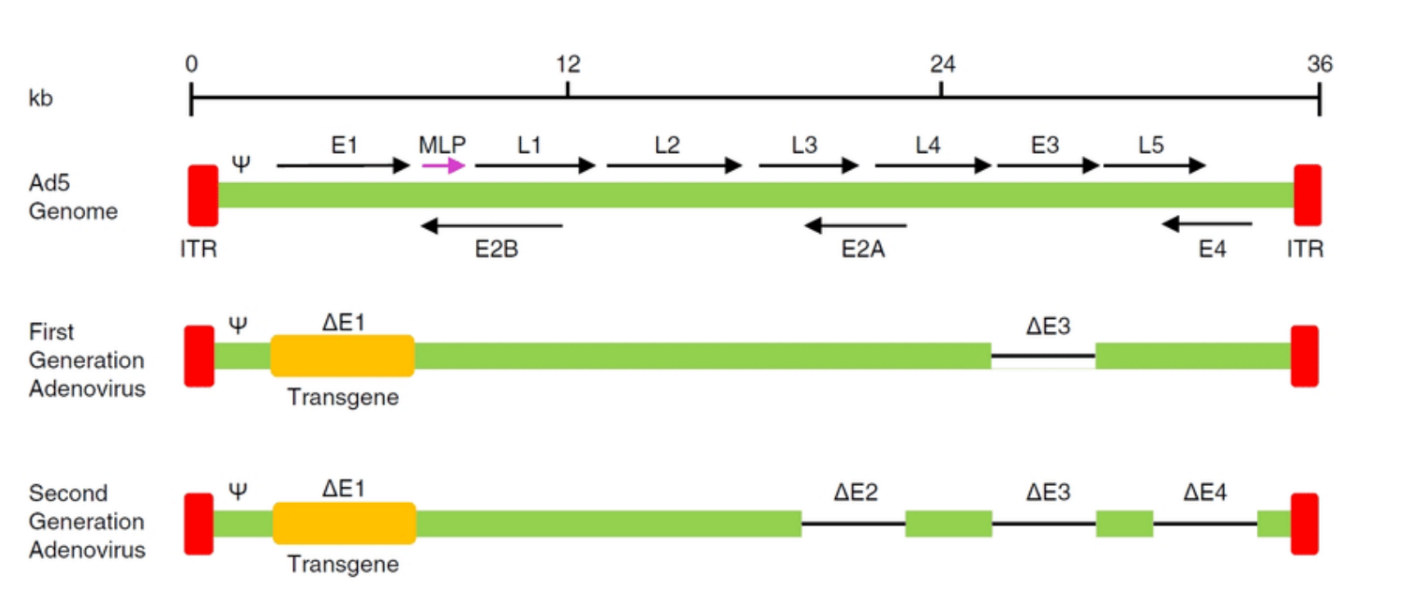
What are ITR?
ITR (Inverted Terminal Repeat):
Function: A sequence found at the ends of the viral genome (in both adenoviral and AAV vectors). It is crucial for viral genome replication and packaging into viral particles.
Role in Vectors: In AAV vectors, ITRs are essential for the virus to replicate in the host cell and for the packaging of the therapeutic gene into viral particles.
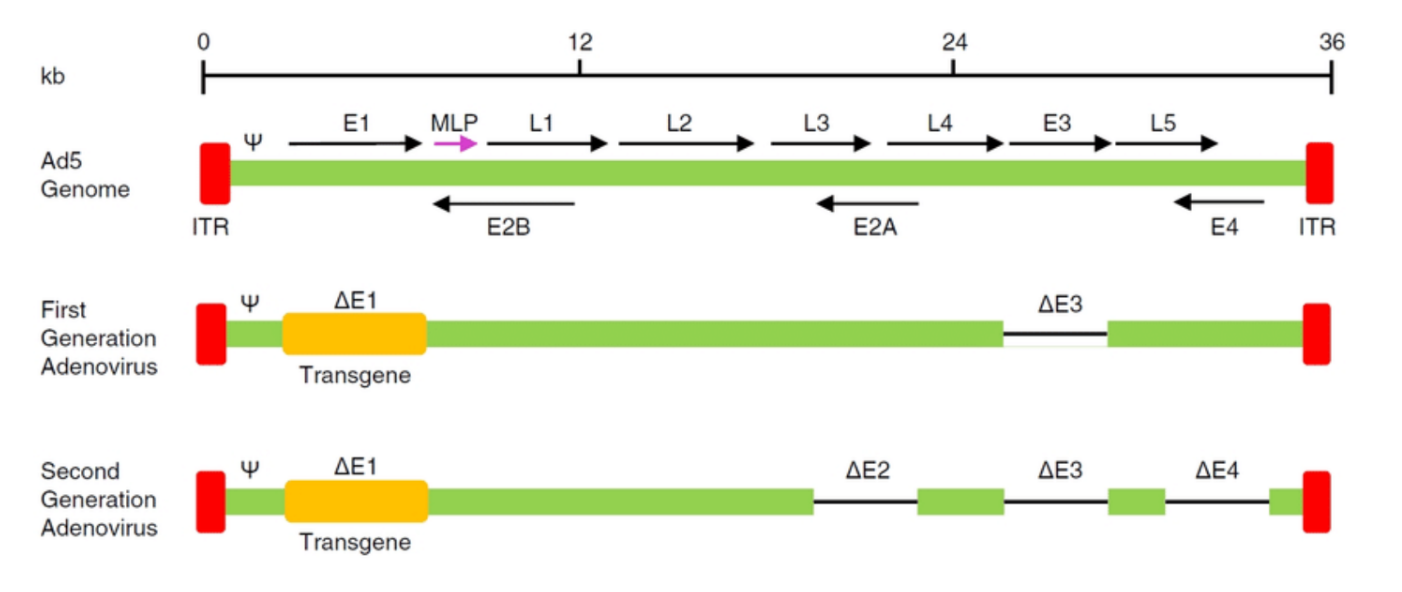
E2A (E2A Transactivator):
Function: A transcription factor that can activate the expression of genes from specific viral vectors.
Role in Vectors: In some systems (like adenoviral vectors), E2A might be used to help activate expression from certain promoters or facilitate the replication of the vector.
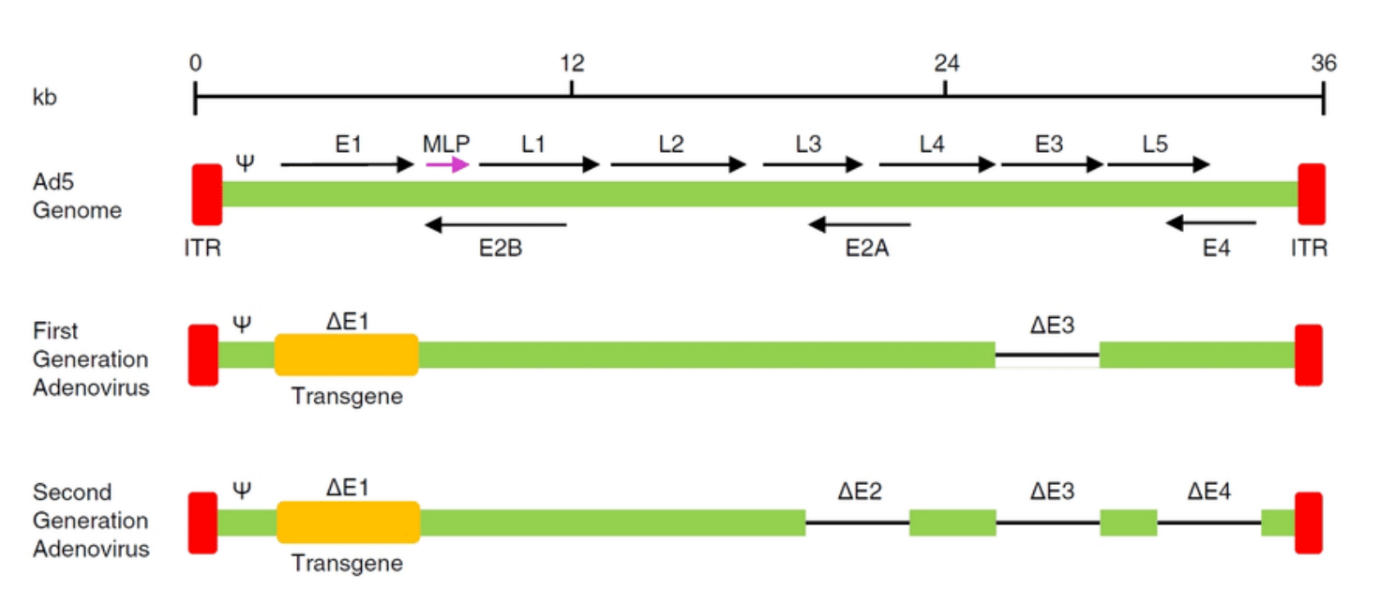
What is E2B?
E2B refers to a part of the E2 region of the AAV genome, which includes the AAV Rep (Replication) proteins.
The E2B region specifically encodes a key protein involved in the replication of the AAV genome.
Function of E2B:
The E2B region is involved in viral DNA replication and packaging of the viral genome into new viral particles.
It includes the Rep proteins which are responsible for replication, regulation, and packaging functions within the viral lifecycle.
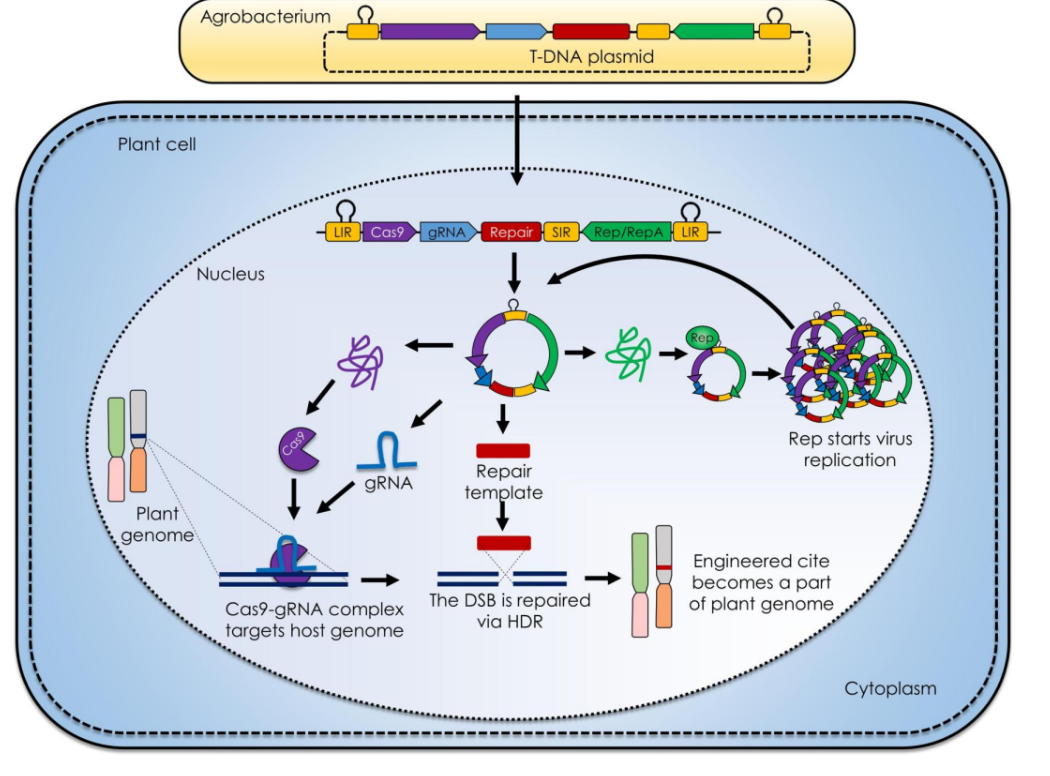
Explain this diagram
T-DNA Plasmid Delivery:
Agrobacterium transfers a T-DNA plasmid into the plant cell. The plasmid contains Cas9, gRNA, a repair template, and viral replication elements (Rep/RepA from geminiviruses).Replicon Amplification:
Inside the nucleus, the Rep protein triggers viral-like replication of the plasmid, boosting the amount of editing components.CRISPR Activity:
Cas9 and gRNA form a complex that cuts the plant genome at a target site.Gene Editing via HDR:
The repair template guides homology-directed repair (HDR), precisely inserting new DNA.Stable Integration:
The edited DNA becomes a permanent part of the plant genome.
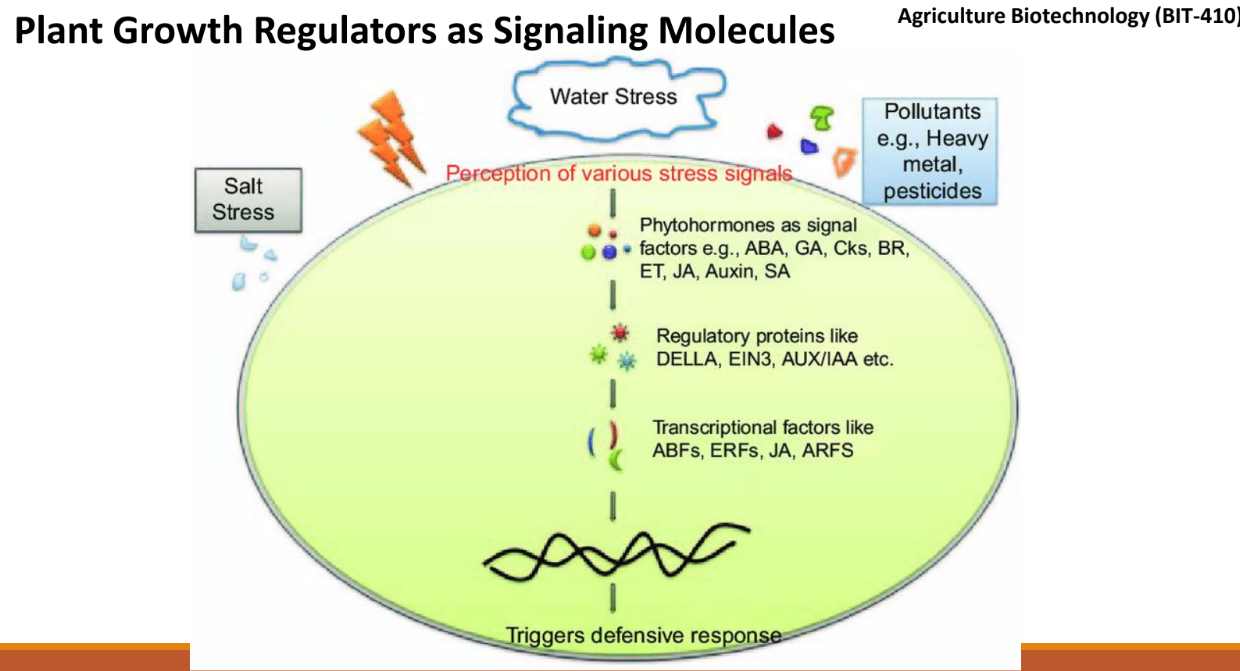
Explain this diagram
Stress is detected by the plant.
Phytohormones (e.g., ABA, GA, JA, Auxin) act as messengers.
Regulatory proteins (e.g., DELLA, EIN3) process the signal.
Transcription factors (e.g., ABFs, ERFs, ARFs) activate specific genes.
This triggers a defensive response, helping the plant survive stress.
What are brassinosteroids?
🌱 Plant growth and development
(e.g., cell elongation, division, and vascular tissue formation)🛡 Stress responses
BRs help plants tolerate abiotic stresses like:Salt stress
Drought
Cold
Heavy metal toxicity
🔄 Crosstalk with other hormones
BRs interact with hormones like ABA, Auxin, and GA to fine-tune growth vs. defense responses.
What are strigolactones?
Root development
– Promote root hair growth and improve nutrient uptake (especially under phosphate starvation).Symbiosis
– Attract arbuscular mycorrhizal fungi, aiding in nutrient exchange.Stress response
– Help plants adapt to nutrient deficiency and abiotic stress like drought and salt
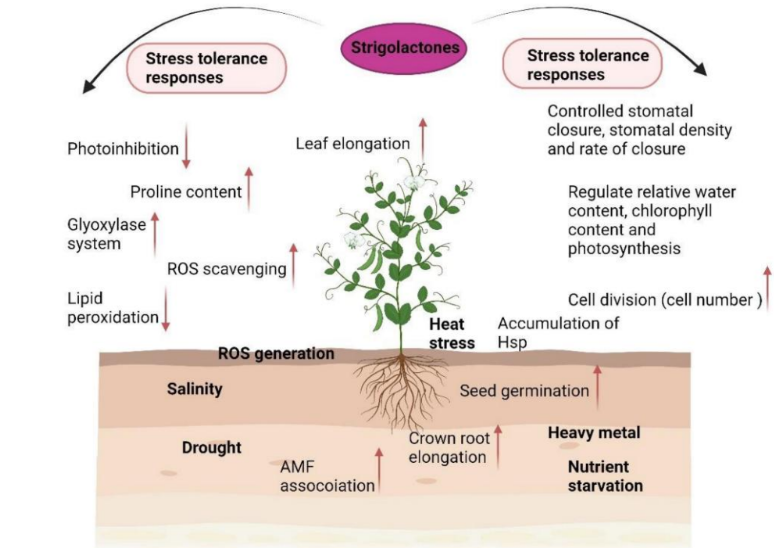
Explain this diagram (above ground)
Above-ground (shoot) responses:
Reduce stress damage:
↓ Photo-inhibition (protects photosynthesis machinery)
↑ ROS scavenging (reduces oxidative damage)
↓ Lipid peroxidation (protects membranes)
↑ Glyoxylase system and proline content (osmoprotection and detoxification)
Support growth:
↑ Leaf elongation
↑ Cell division (↑ cell number)
↑ Accumulation of Hsp (heat shock proteins)
Water management:
Regulate stomatal closure, density, and rate → helps conserve water
Maintain chlorophyll, water content, and photosynthesis
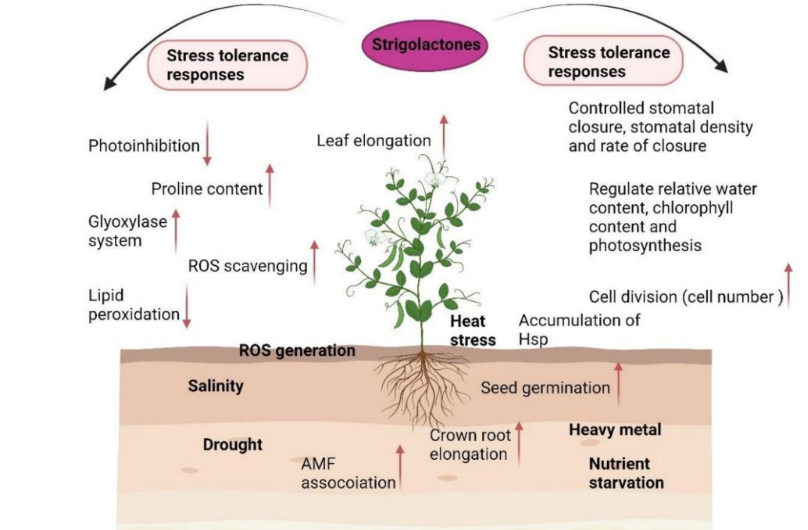
Explain this diagram (below ground)
🔽 Below-ground (root) responses:
↑ Seed germination
↑ Crown root elongation
↑ Association with AMF (arbuscular mycorrhizal fungi) — improves nutrient uptake
Help tolerate:
Salinity
Drought
Heat stress
Heavy metal toxicity
Nutrient starvation