IR Spectroscopy
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
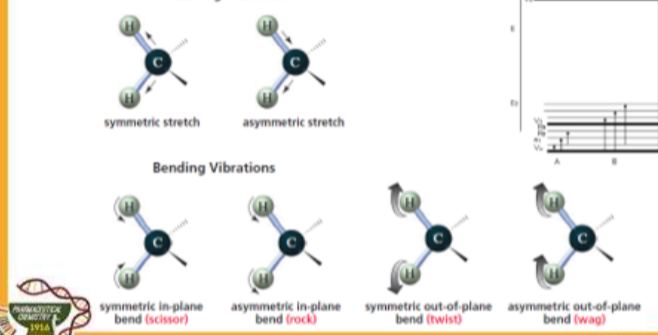
Bond Stretches and Bends
Stretches:
Symmetric Stretch
Asymmetric Stretch
Bends:
Symmetric In-Plane Bend (Scissor)
Asymmetric In-Plane Bend (Rock)
Symmetric Out-of-Plane Bend (Twist)
Asymmetric Out-of-Plane Bend (Wag)
Hooke’s Law
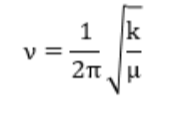
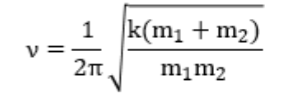
μ = corrected mass (m1*m2)/m1+m2
so
And divide by speed of light in cm to get wavenumber (3.00 × 1010)
Effect of Mass on Wavenumber
higher mass = smaller wavenumber
Lighter atoms absorb at higher wavenumbers
Effect of Bond Order on Wavenumber
Higher bond number = stronger spring = larger k
larger k = higher wavenumber
Higher bond numbers absorb at higher wavenumbers
Effect of Resonane on Wavenumber
depends
If resonance removes double bond and gives higher single-bond character then the resonance
weakens spring
lowers k
reduces wavenumber
Effect of Hydrogen Bonding on Wavenumber
pulls on OH bonds (or NH/etc), making the bonds weaker causing minimal differences
Hydrogen bonding “removes” electronegativity, leading to lower wavenumber (slightly)
different if dilute or concentrated hydrogens
Also explains some broad peaks; H-bonding changes bond length, and so variable stretch
Effect of Hybridization on Wavenumber
More s-character means shorter bonds meaning stronger springs = higher wavenumber
Wavenumber: sp > sp2 > sp3
Important Note about Amine and Amide Stretches
Primary amines/amides have a double peak at their NH stretch (because they have 2 NHs)
Secondary amines/amides only have a single peak
Tertiary amines/amides have no NH stretch, and must be found in the fingerprint region