BIOPHYSICS FORMULAE
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
71 Terms
First derivative of a function f(x) x small change in dx
dy = f'(x)dx
To find slope or gradient
tan ⍺ = Δy/Δx
Partial differentiation
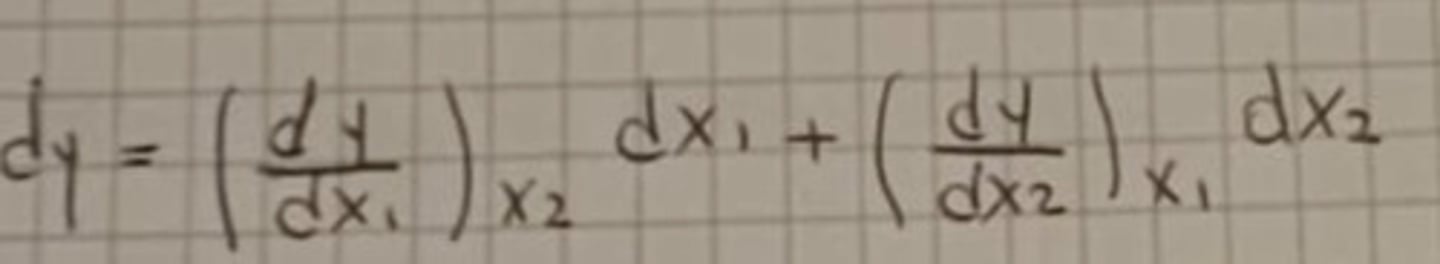
Chemical potential
dG = Tot differential
p, T, n, j = Constant variable
dni = Differential with this K
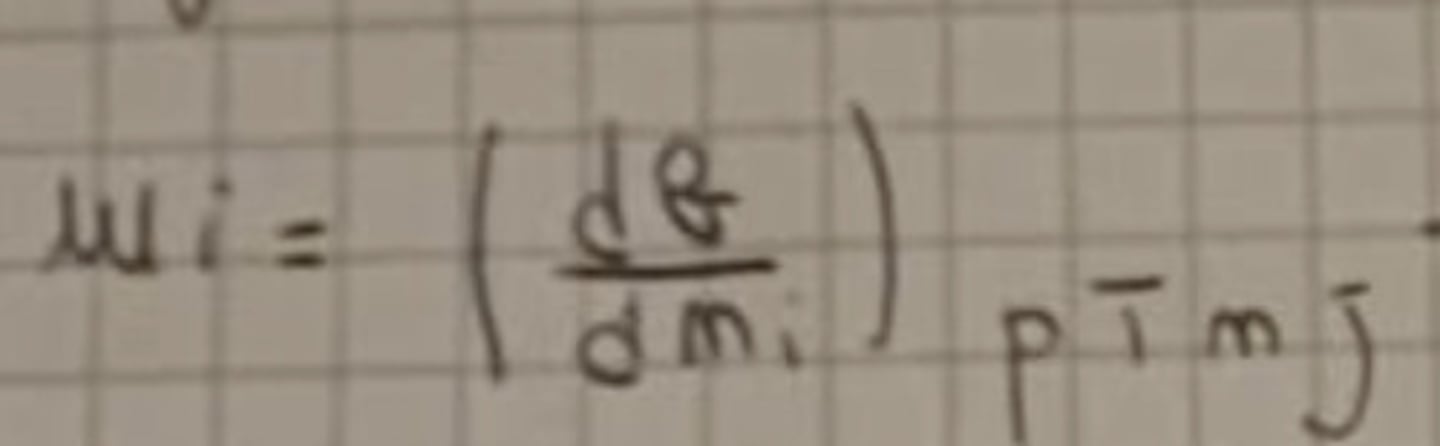
Work
W = F x dx
F = Force
dx = Displacement
First law of T. for isolated system
dU = 0
Mathematical formulation of 1° law of T. for closed system
dU = dQ + dW
Q = Heat
U = Internal energy
W = Work
Internal energy
Ek = Kinetic energy
Ep = Potential energy
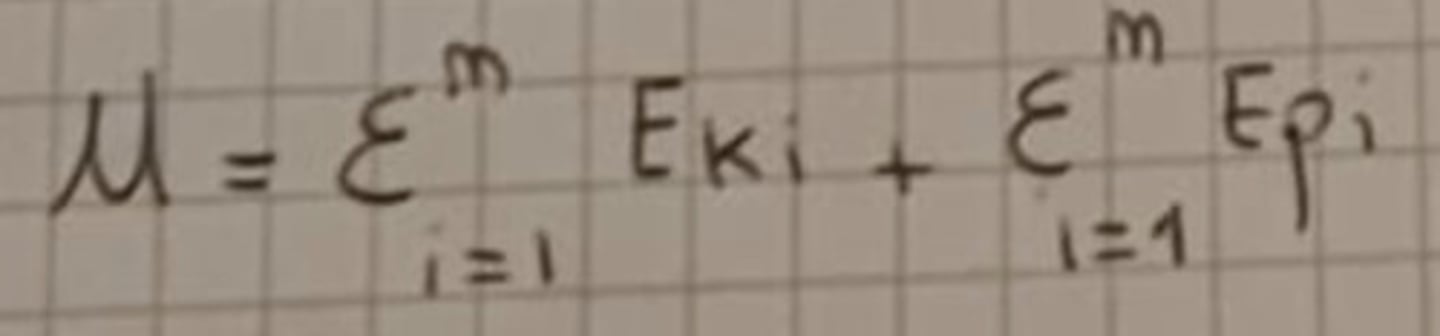
Thermodynamic work
dW = -PdV
P = Pressure
V = Volume
Electric work
dW = ɸdq
ɸ = Electrical potential
dq = Amount of charge
Osmotic work
dW = μdn
2° law for reversible process
dS = S2 - S1 = dQ/T
S = Entropy
Q = Heat
T = Temperature
2° law for irreversible process
dS > dQ/T
S = Entropy
Q = Heat
T = Temperature
2° law during reversible process (isolated system)
dS = 0
2° law during irreversible process (isolated system)
dS > 0
2° law for closed and open system
dS < 0 dQ < 0
Boltzmann equation for entropy
S = k ln W
S = Entropy
k = Boltzmann constant
W = Thermodynamic probability
Boltzmann constant (k)
k = R/Na
R = Constant of perfect gas
Na = Avogadro's number
Perfect gas law
PV = nRT
P = Pressure
V = Volume
n = Number of mole
R = Universal gas constant
T = Temperature
Shannon equation of information
I = k ln P
I = Information
k = Boltzmann constant
P = Mathematical probability
Mathematical probability
P = n. of favorables cases/ Greatest n. of cases
Heat of reaction
ΔH = H (products) - H (reagents)
Potential energy
Ep = mgh
m = Mass
g = Acceleration of gravity
h = Height
Work
W = -dEp
Ep = Potential energy
Force
Fdx = -dEp (Force = - gradient of Ep)
F = -(dEp/dx)
Electrical potential
ɸ = Ep/q
Driving force (electrical force)
F = -q x dɸ/dx
q = Charge
dɸ/dx = Electrical potential gradient
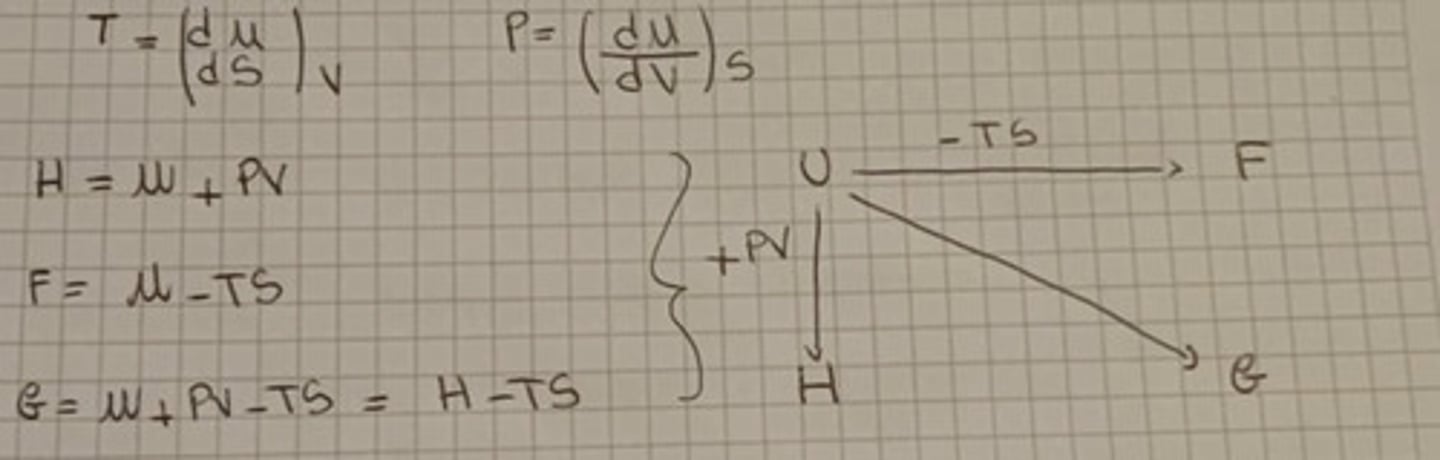
Combined Law of Thermodynamics
dU = TdS - PdV
U = Internal energy
S = Entropy
P = Pressure
V= Volume
1° law for an open system
dU = dQ + dW + μdn (dU = TdS - PdV + μdn)
U =Internal energy
Q = Heat
W = Work
μ = Chemical potential
n = number of particles
Chemical potential
μ = μ0 + RT ln C
Change in free energy
dG = μdn
μdn = Change in electrochemical potential
Electrochemical potential
dμ* = dμ0 + RT ln C2/C1 + z x F x (ɸ2 - ɸ1)
F = Faraday's constant
z = Ion valency
(ɸ2 - ɸ1) = Potential difference
Faraday's constant
F = eNa
e = Generic charge
Na = Avogadro's number
Charge
q = e x z
e = Elementary charge
z = Atomic number
Flux
J = Cux
C = Concentration
u = Mobility
x = Foce
Flux of matter
J^m = -D x dC/dx
D = Diffusion coefficient
dC/x = Concentration gradient
Flux of heat
J^Q = - k x dT/dx
K = Constant
dT/dx = Temperature gradient
Force
F = m x a
m = Mass
a = Acceleration
Mobility (uncharged particles)
u = v/x
v = Velocity
x = Force
Relationship btw Force and Flux (Onsager coefficient)
J = Lx
L = Onsager coefficient
x = Force
Phenomenological equation
Jm = Lm1x1 + lm2x2 + ......... + Lmnxn
Jm = Number of flux
n = Number of driving force
Total entropy balance
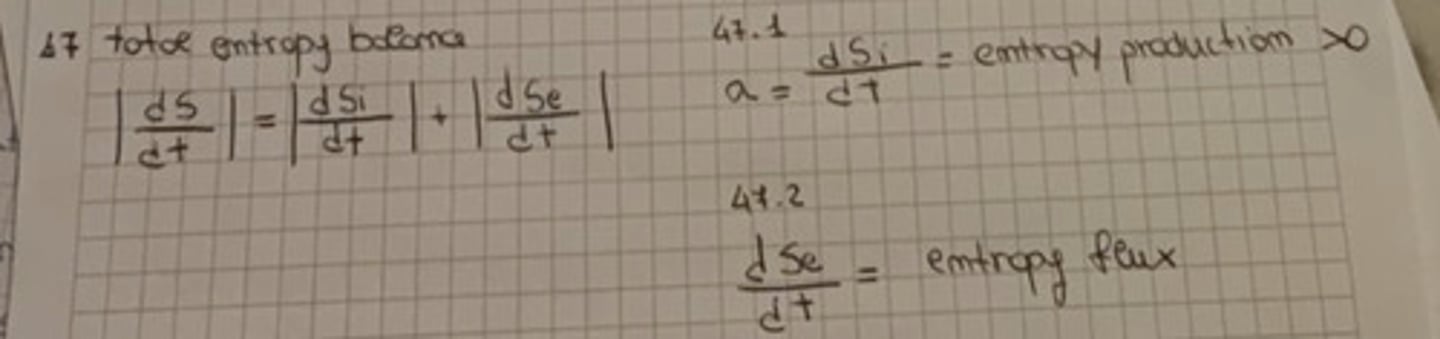
Criteria of coupling flux
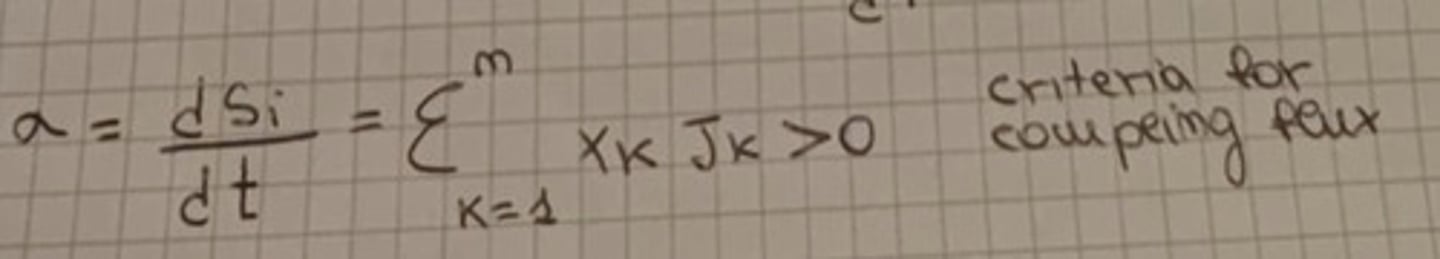
Stock's law (Frictional force)
Fd = 6πηrv
η = Viscosity
r = Radius
v = Velocity
Fick's law for diffusion
dm/dt = -DA dC/dx
dm/dt = Mass flow
D = diffusion coefficient
A = Area
dC/dx = Coefficient gradient
Flux of matter (Fick's)
dm/dtdA
m = Mass
t = Time
A = Area
Coefficient of diffusion
D = uRT
u = mobility
R = Universal gas constant
T = Temperature
Fick's for free diffusion of non-charged particles
J = -D dC/dx ==> J = -uRT dC/dx
D = Diffusion coefficient
u = mobility
R = Universal gas constant
T = Temperature
Electric field
E = F/q
F = Force
q = Charge
Electric potential gradient
E = - dɸ/dx
1 mol of ions
F = - z x F x dɸ/dx
z = Ion valency
F =Faraday's constant
dɸ/dx = Electric potential gradient
Free diffusion of charged particles (Drift)
J = - cuzF dɸ/dx
c = Concentration
u = Mobility
z = Ion valency
dɸ/dx = Electrical potential gradient
Nernst-Plank molar flux equation
J = - uRT dC/dx - cuzF dɸ/dx
u = Mobility
R = Universal gas constant
T = Temperature
dC/dx = Concentration gradient
c = Concentration
z = Ion valency
F = Faraday's constant
dɸ/dx = Electrical potential gradient
Partition coefficient
k = Cme/Ce = Cmi/Ci
Concentration intracellular and extracellular
Permeability
P = Dk/d
D= Diffusion coefficient
k = Partition coefficient
d = Membrane thickness
Fick's law for simple diffusion
J^m = -PdC
P = Permeability
C = Difference in concentration
Pousille's law
πr^4dP/8ηl
r = Radius
P = Pressure
η = Viscosity
l = Length
Michealis-Menten equation
J = Jmax x C/Km + C
J = Flux
Jmax = Maximum flux
C = Concentration
Km = Michealis-Menten constant
Henderson equation (Diffusion potential)
u = Mobility
R = Universal gas constant
T = Temperature
z = Ion valency
C = Concentration
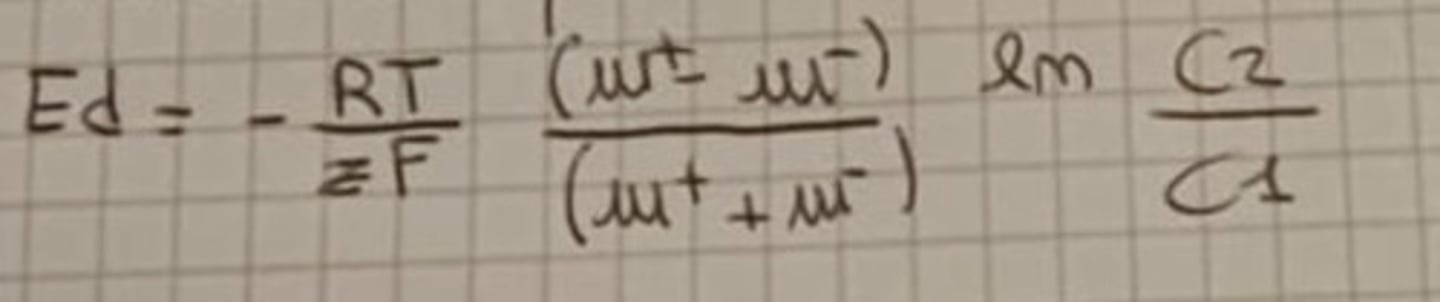
Nernst equation (Equilibrium potential)
Em = - RT/zF ln C2/C1 ==> (Em = - 60 log10 C2/C1)
R = Universal gas constant
T = Temperature
z = Ion valency
C = Concentration
Donnan equilibrium
[K+]A x [Cl-]A = [K+]B x [Cl-]B
Resting membrane potential (Goldman equation)
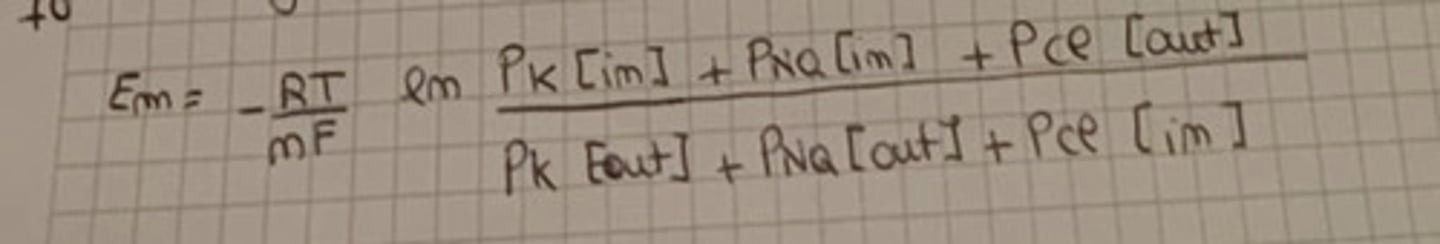
Thomas equation
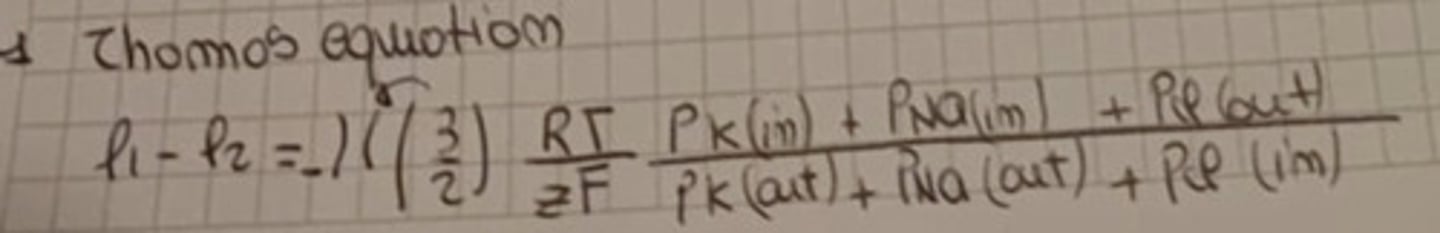
Electric current
I = 1/R x V = gV
R = Resistance
V = Voltage
g = Conductance
Conductance
g = 1/R
R = Resistance
Ohm's law
V = I x R
I = Electric current
R = Resistance
Fourier's law
dQ/dt = - k dT/dx
k = constant
dT/dx = Temperature gradient
mobility (charged partcles)
u = v/E
Osmotic pressure
Posm = cRT
c = Concentration
R = Universal gas constant
T = Temperature
Einstein equation
E = mc^2
m = Gravitational mass
c = velocity of the light
Newton's law for viscosity
τ = μ x du/dy
τ = Shear stress
μ = Viscosity
du/dy = Rate of shear deformation