organic chemistry lect2
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Alkanes formula
CnH2n+2
Cyclo alkanes formula
CnH2n
Alkenes formula
CnH2n
(Same as cyclo alkanes)
Cyclo alkenes formula
CnH2n-2
Alkynes formula
CnH2n-2
Aromatic compounds
Hydrocarbons that contain a special type of rings.
Most common example of aromatic compounds?
A benzene ring
Primary carbon
Attached to only one carbon
Secondary carbon
Attached to two carbons
Tertiary carbon
attached to three carbons
How are hydrogen referred to ?
1^0 2^0 3^0
According to the type of carbon they are bonded to.
Functional groups
Specific groups of atoms within molecule that are responsible for the characteristic chemical reactions of those molecules.
Phenyl group (Ph— or Ar—)
C6H5
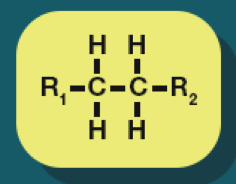
Alkane
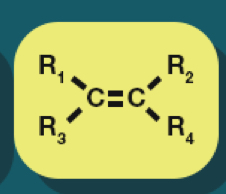
Alkene
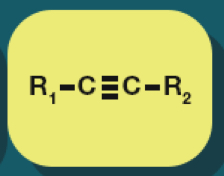
Alkyne
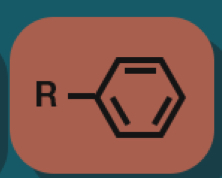
Arene

Haloalkane
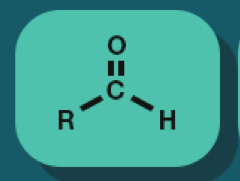
Aldehyde
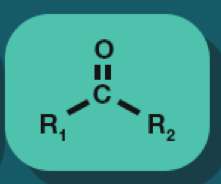
Ketone
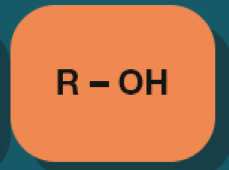
Alcohol
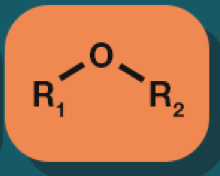
Ether
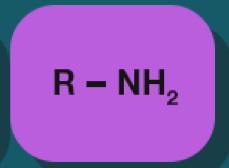
Amine
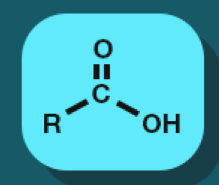
Carboxylic acid
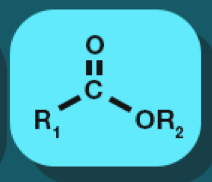
ester
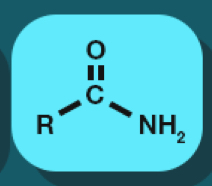
Amide
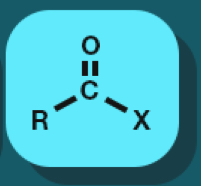
Acyl halide