HYMS B9, W2
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
common pneumonia causing microorganism
Streptococcus pneumoniae is the most common
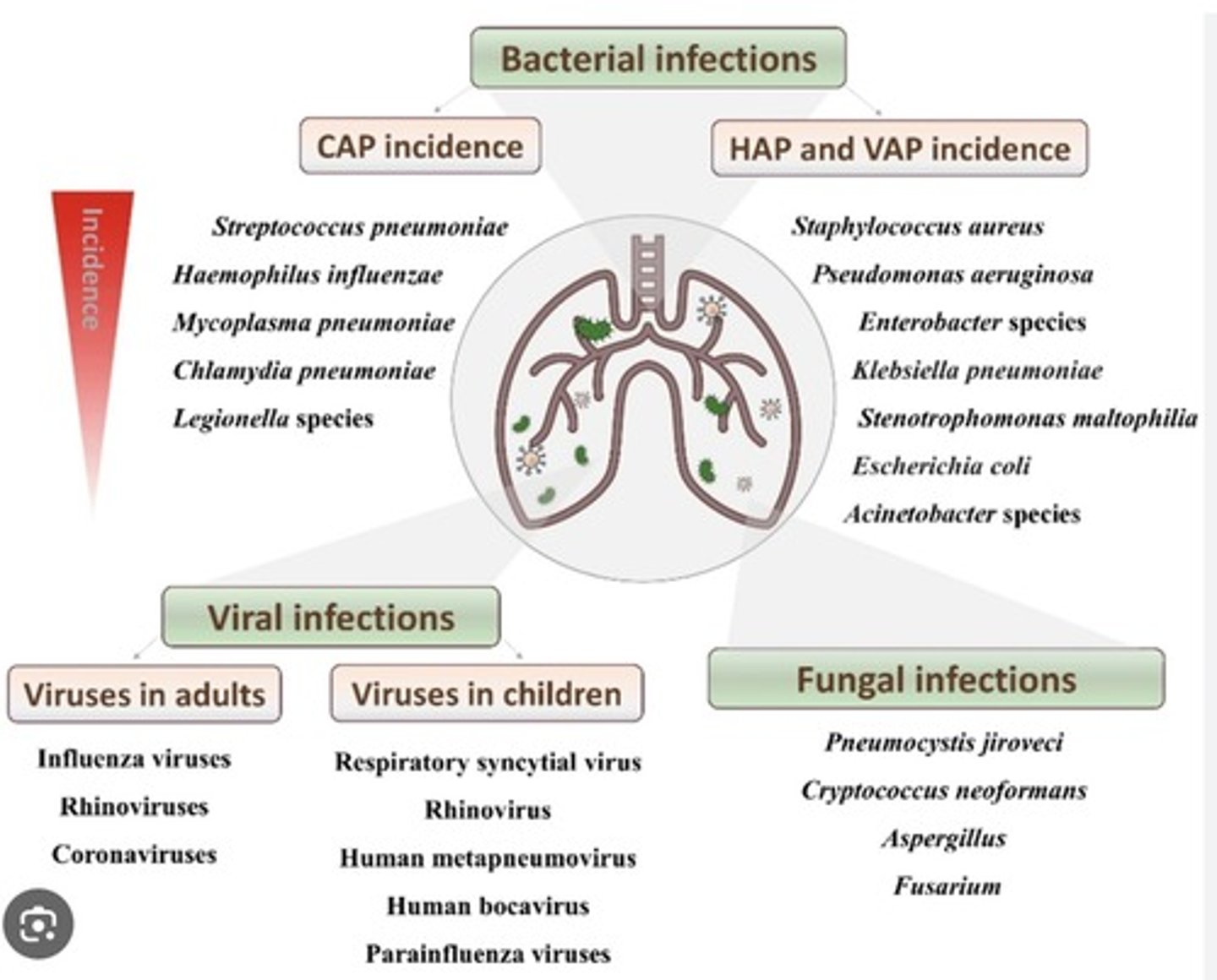
most common bacterial CAP microorganism
- Strep pneumoniae
- Haemophilius influenzae
- Mycoplasma pneumoniae
- Chlamydia pneumoniae
- Legionella species
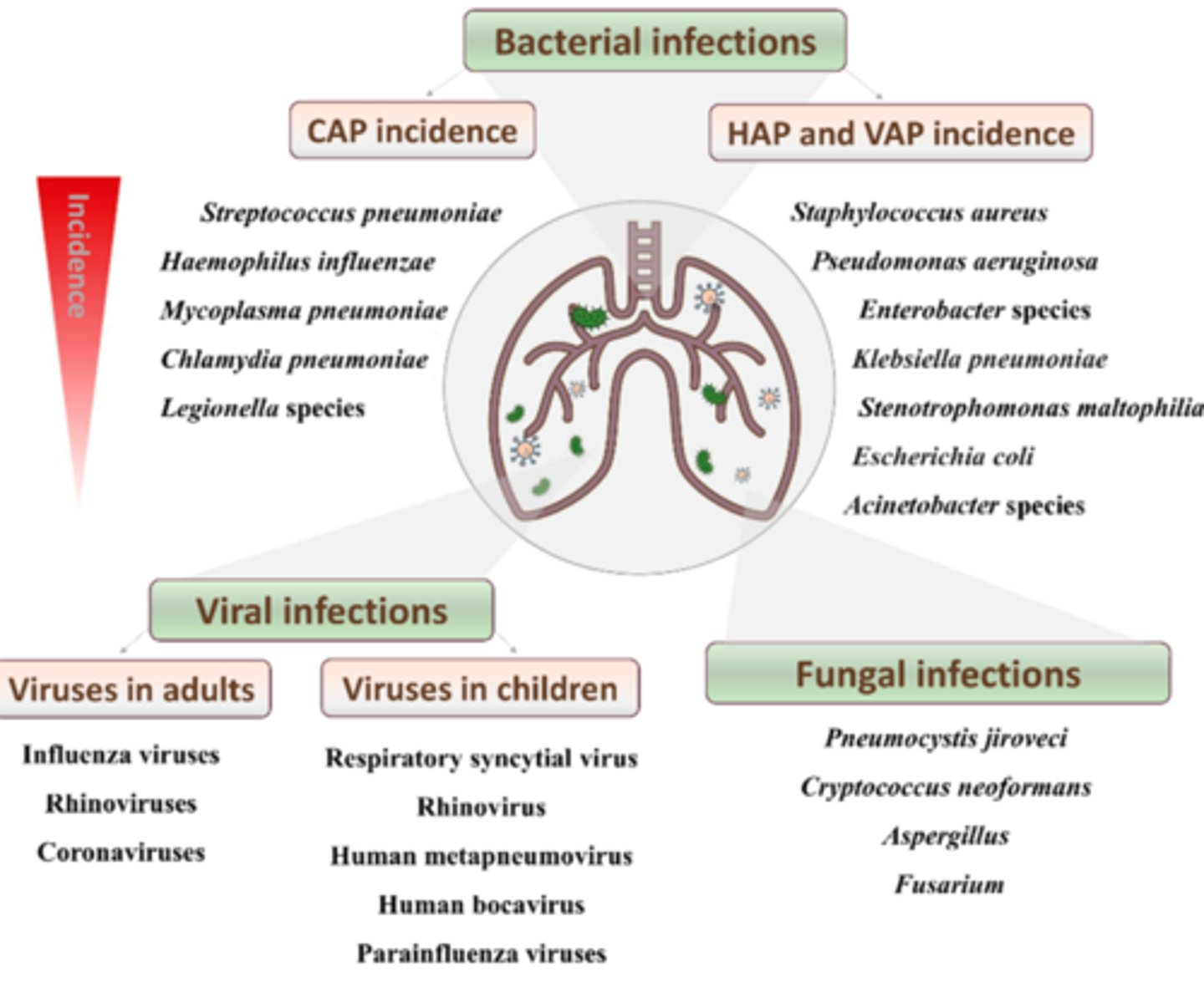
most common bacterial HAP and VAP microorganisms
- Staph aureus
- Pseudomonas aeruginosa
- Enterobacter species
- Klebisella pneumoniae
- E.coli
most common fungal causes of pneumonia
- Pneumocystis jiroveci
- Aspergillus
most common viral causes of pneumonia in children
- respiratory syncytial virus
- rhinovirus
- human metapneumovirus
- human bocavirus
- parainfluenza virus
most common viral causes of pneumonia in adults
- influenza viruses
- rhinoviruses
- coronaviruses
risk factors for bacterial pneumonia
Lung diseases, such as asthma, bronchiectasis, cystic fibrosis, or COPD, also increase your pneumonia risk.
Other serious conditions, such as malnutrition, diabetes, heart failure, sickle cell disease, or liver or kidney disease
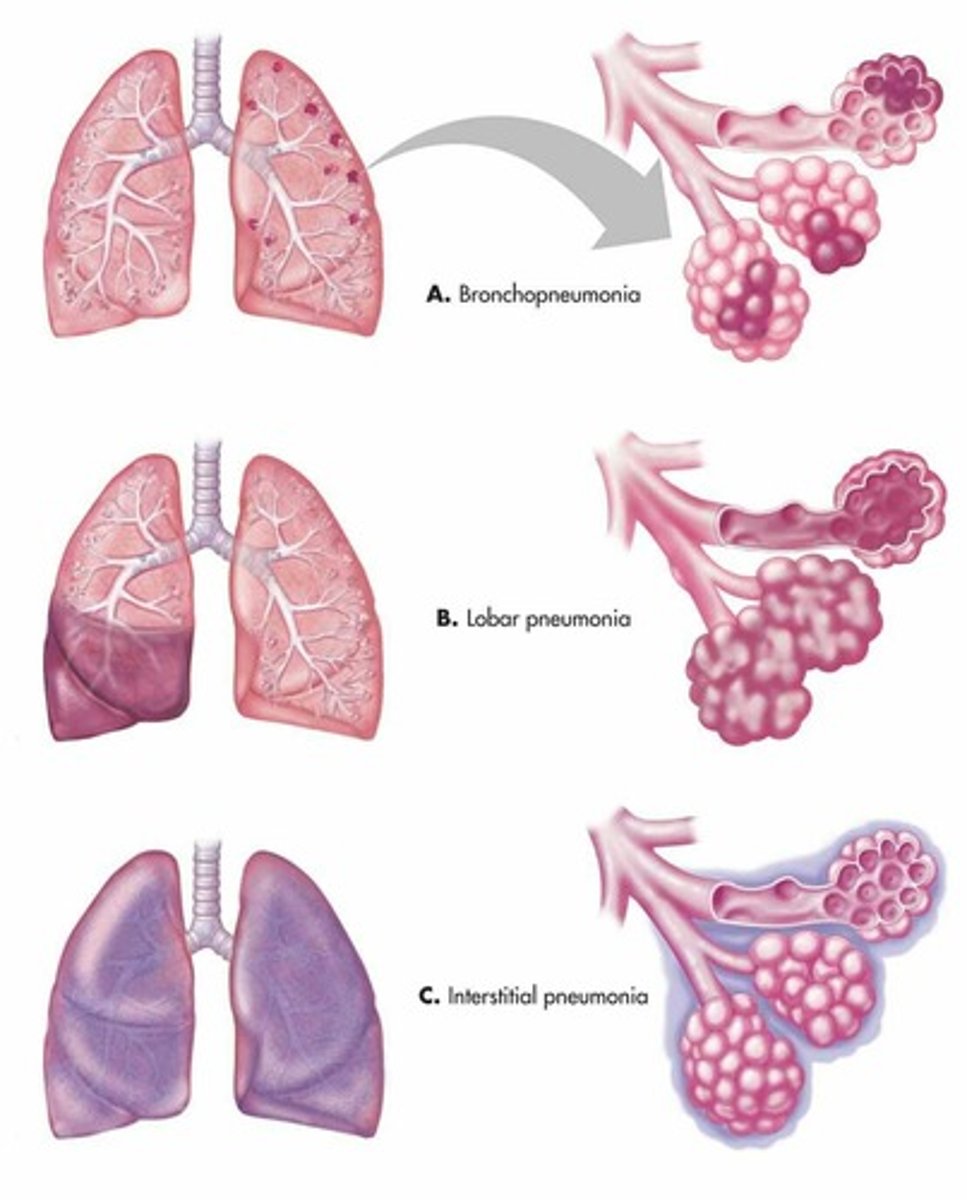
pathophysiology of bacterial pneumonia (lobar)
1. Congestion. Pathogen enters the lower respiratory tract (bacteria, viruses, or fungi). Inflammation occurs so blood vessels become more permeable and alveoli fill with excess fluid.
2. red hepatisation - exudate fills air spaces making them more solid. creates consolidation of lung.
3. grey hepatisation - RBC in exudate breaks down changing the colour.
4. resolution - exudate gets digested / coughed up or broken down.
pneumonia categories
•Community-acquired pneumonia (CAP)
•Hospital-acquired pneumonia (HAP)
•Ventilator-associated pneumonia (VAP)
OR
bronchiole pneumonia (lobular)
atypical / interstitial pneumonia
lobar pneumonia (affects lung parenchyma, mainly alveoli)
penicillin mechanism of action
Penicillin pass through porins of gram negative bacterial cell wall. The penicillin then binds to penicillin binding protein linked the cell membrane to be activated. Active penicillin binds to and inactivate transpeptidase enzyme. As a result peptidoglycans NAM and NAG sugars are not cross-linked and the cell wall collapse
Macrolides mechanism of action
Inhibit protein synthesis by binding to the 50s subunit (of the 70s ribosome), preventing translation.
bacteriostatic effect
what are the two innermost layers of the meninges called?
leptomeninges
made up of the arachnoid and pia mater
inflammation of the brain and meninges is called...
meningoencephalitis
cephalosporins class
β-lactam antibiotics
cephalosporins mechanism of action
Beta-lactam rings in the antibiotic bind to peptidoglycan transpeptidase (a penicillin binding protein that creates cross links in the peptidocyglcan cell wall). Binding inhibits its normal activity. the bacteria can't synthesis a cell wall and therefore die.
What role does the gut microbiome play in the control of homeostatic mechanisms in the body?
- protects against pathogens
- synthesis of vitamins (e.g. vit k)
- immune system development
- promotes intestinal angiogenesis
- promotes fat storage
- SCFA production by fermentation of dietary fibre
- modulation of CNS
How is fever created?
- inflammatory response triggered
- IL-1, IL-6 and TNF are released. these are known as pyrogenic cytokines
- these increase the synthesis of prostaglandin E2 in the pre optic area of the hypothalamus
- PE2 raises core body temperature by vasoconstriction, thermogenesis (in fat and muscle cells) and sometimes shivering.
rigor
a sudden feeling of cold with shivering accompanied by a rise in temperature, often with copious sweating, especially at the onset or height of a fever.
resistant consortia of bacteria meaning
two or more interacting microbial populations that provide microbial protectors against a pathogen.
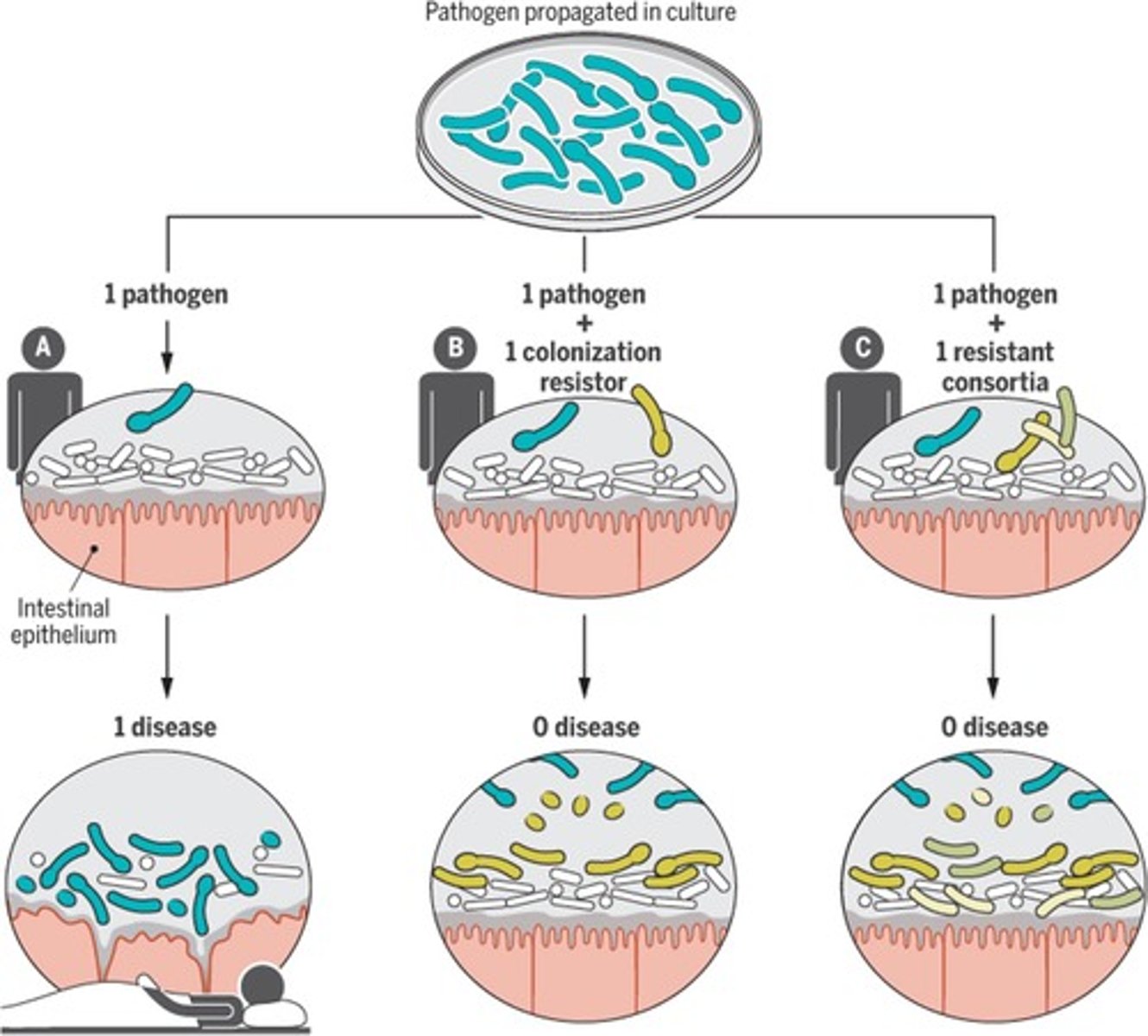
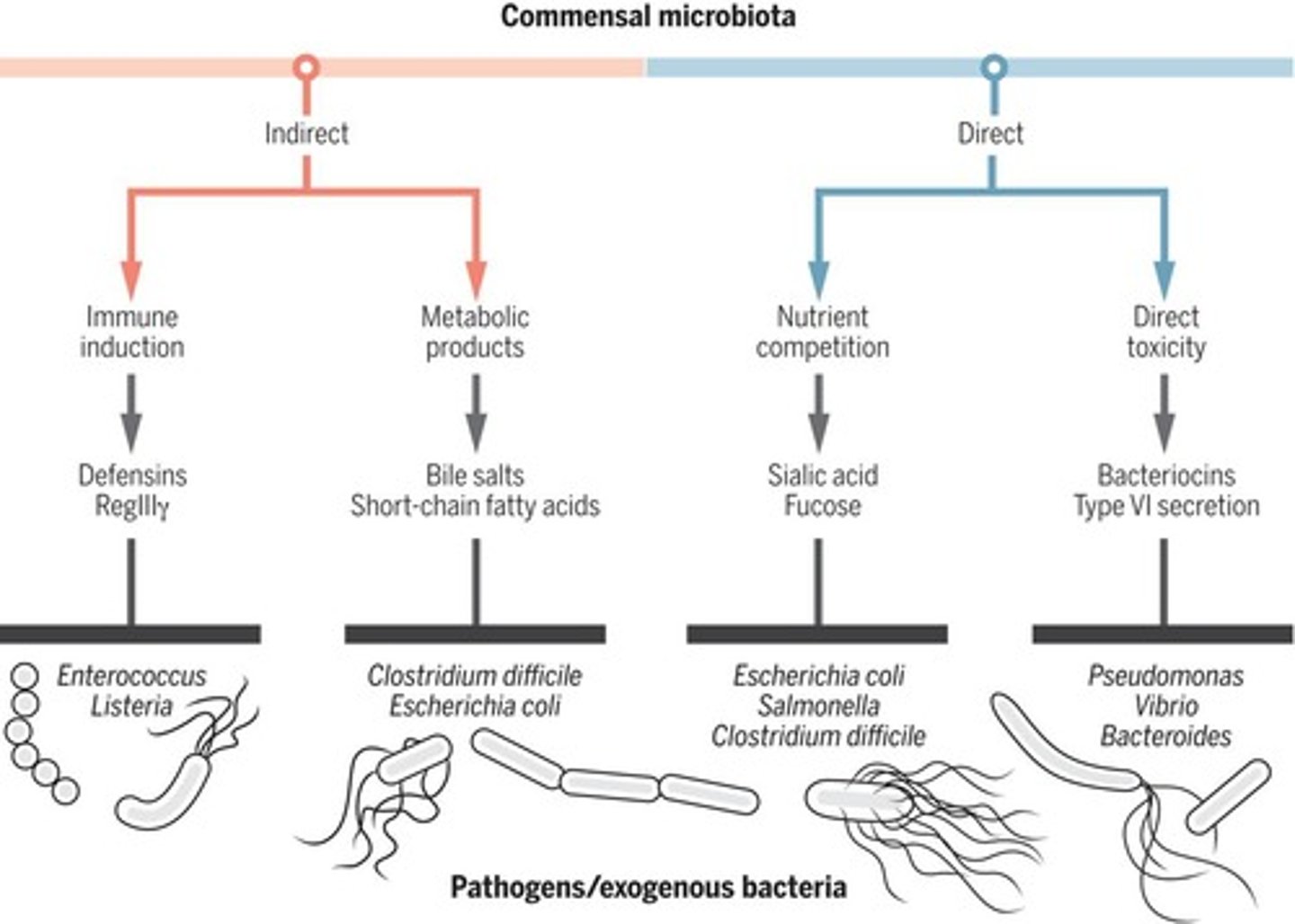
How can intestinal commensal microbiota provide direct colonisation resistance against pathogens?
- nutrient competition
- direct toxicity
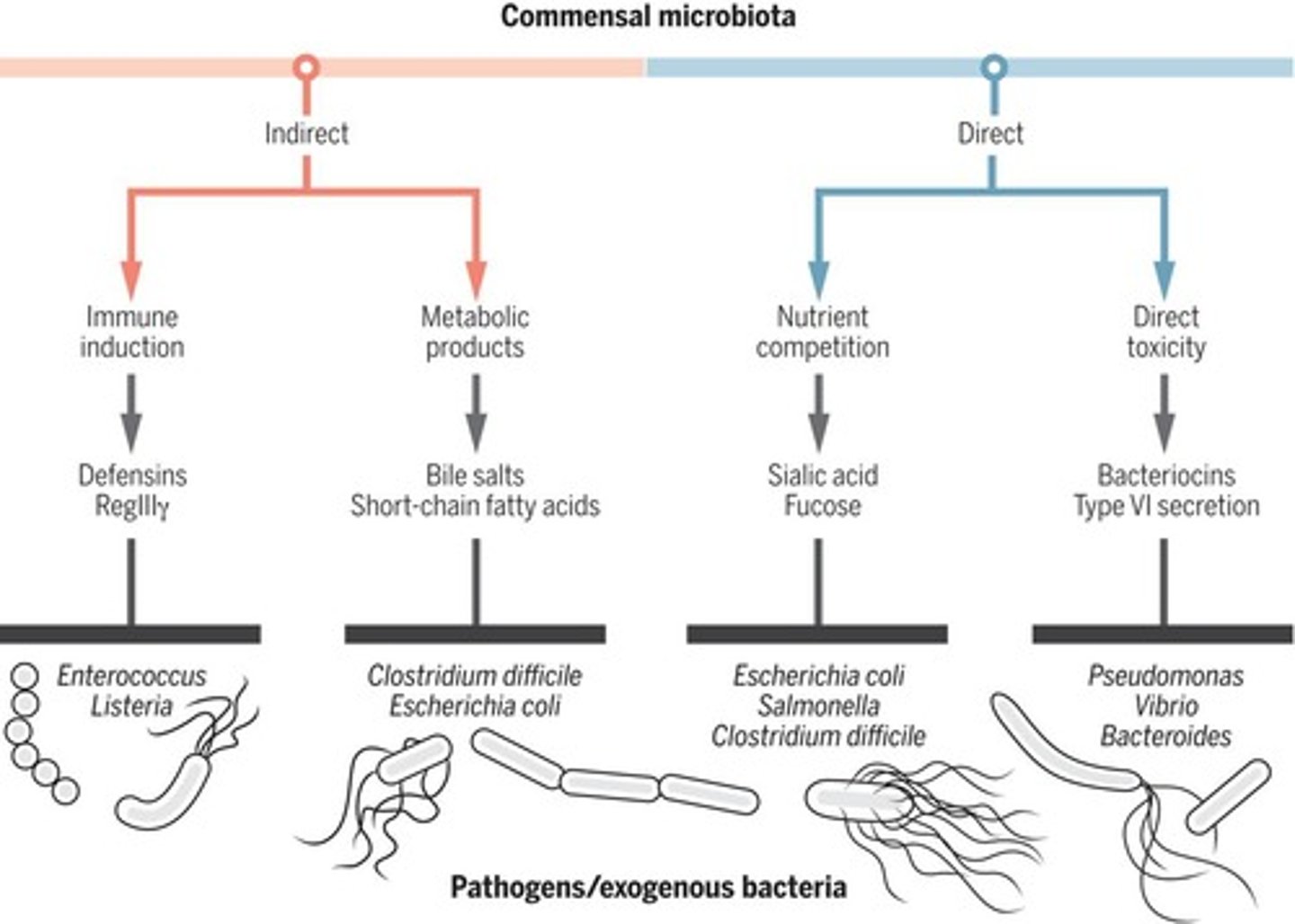
How can intestinal commensal microbiota provide indirect colonisation resistance against pathogens?
- immune induction
- metabolic products
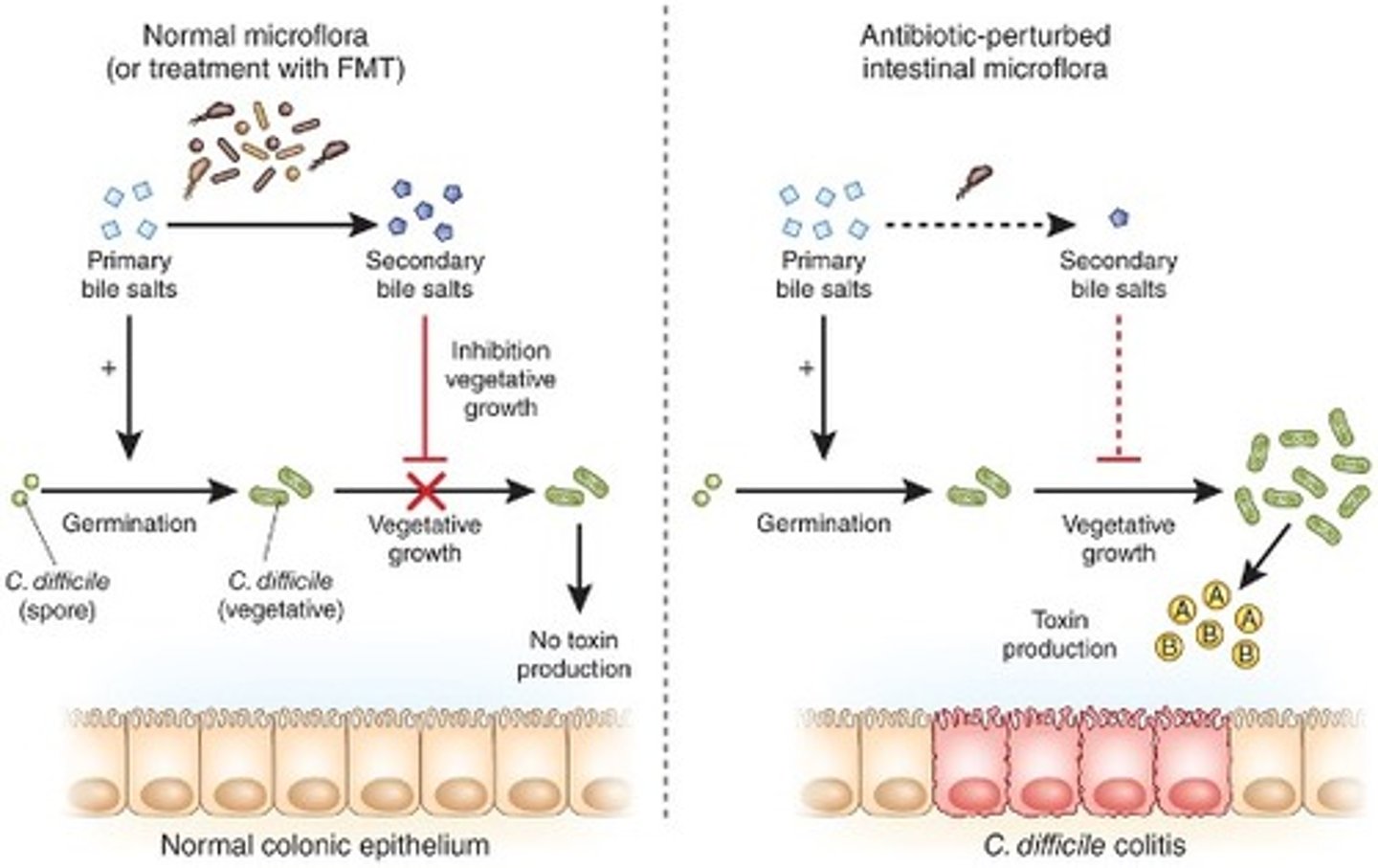
which microorganism often causes antibiotic-associated diarrhoea (pseudomembranous colitis)?
Clostridium difficile
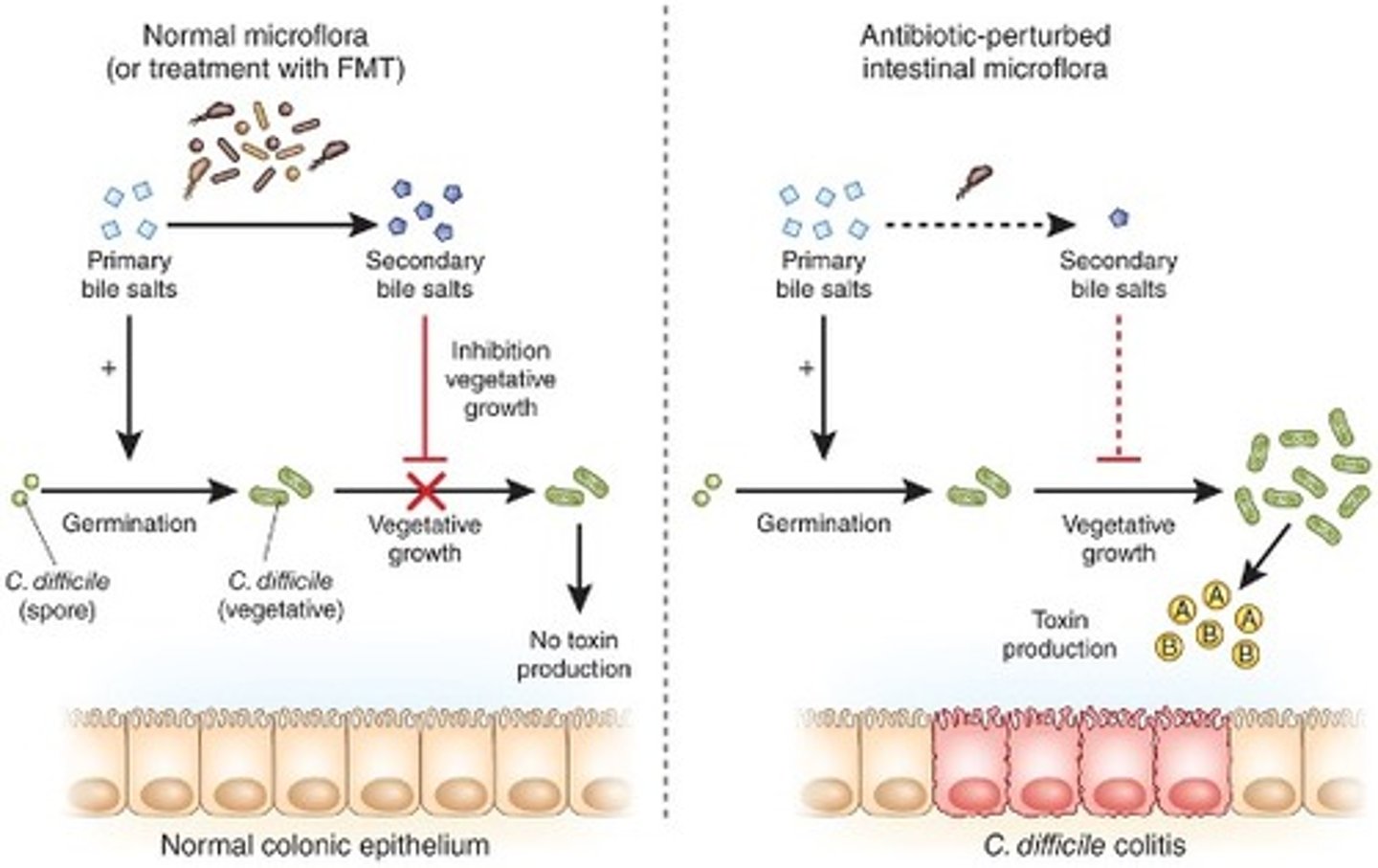
How does Clostridium difficile cause antibiotic-associated diarrhoea?
Flourishes under antibiotic selective pressure
what factors can modify the human microbiome?
age, environment, host genetics, diet, lifestyle, immune response, microbial co-adaption
dysbiosis
- imbalance of normal gut microbiota composition
- these changes harmfully effect the host
prebiotics
non-digestible food that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon, and thus improving the host's health.
what role does the human microbiome have in bacterial pathogenesis?
- Creates competition for nutrients or direct antagonism (produces substances against pathogens).
- commensal bacteria can trigger antibody production against pathogens. also balances pro and anti-inflammatory responses.
- production of metabolites - e.g. production of SCFAs from dietary fibre. these can inhibit growth of some pathogens.
- dysbiosis - antibiotics for example can disturb commensal bacteria allowing pathogenic bacteria to grow.
- aids recovery post infection.
- influence drug efficacy and metabolism
- therapeutics - e.g. fecal microbiota transplants (to treat recurrent C. difficile infections), or making pre/probiotics.
probiotics
living non-pathogenic organisms used as food ingredients to benefit the host's health
What may trigger meningitis?
- infection (e.g. herpes simplex, Neisseria meningitidis)
- auto-immune diseases (lupus)
- adverse reaction to medication (intrathecal therapy)
Neisseria meningitidis is also known as
meningococcus
what are the two routes of infection reaching the meninges?
- direct spread - penetration through skin and skull into meninges
- haematogenous - blood --> endothelium --> CSF. This happens in weakened endothelium or where there is surface proteins for the bacteria to bind to on it.
what is the most common cause of bacterial meningitis in newborns?
Group B Streptococcus
Streptococcus pneumoniae,
Listeria monocytogenes
E. coli
what is the most common cause of bacterial meningitis in children and teens?
Neisseria meningitidis
Streptococcus pneumoniae
what is the most common cause of bacterial meningitis in adults and the elderly?
Streptococcus pneumoniae
Listeria monocytogenes
what are the most common causes of viral meningitis?
enteroviruses (coxsackie)
herpes simplex
HIV
name some common differential diagnoses for meningitis
influenza or other viral illnesses
sepsis
encephalitis
intracranial or spinal absences
subarachnoid haemorrhage
brain or CNS malignancy
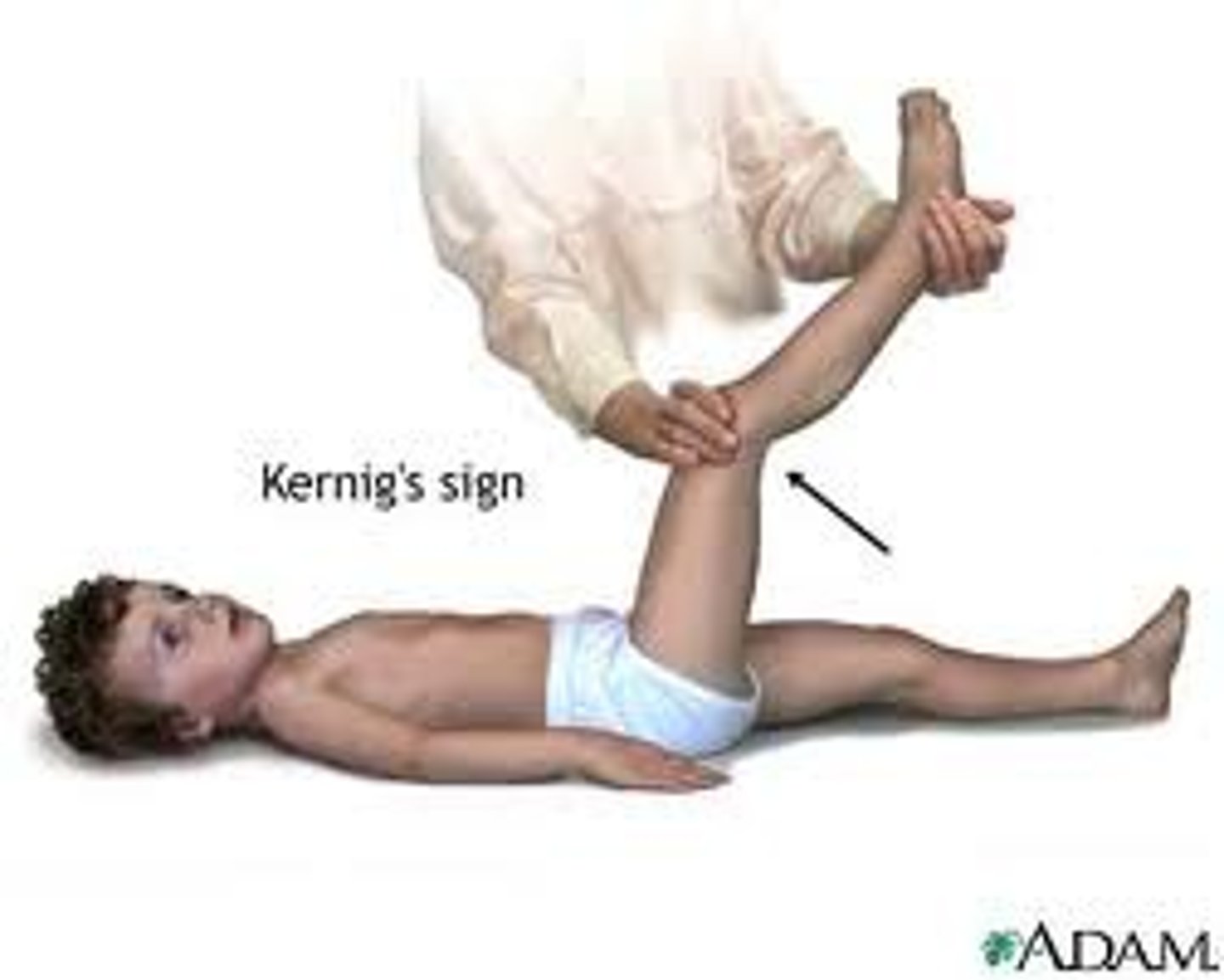
What is Kernig's sign?
test for meningitis
After flexing the hip and knee at 90 degree angles, pain and resistance are noted.
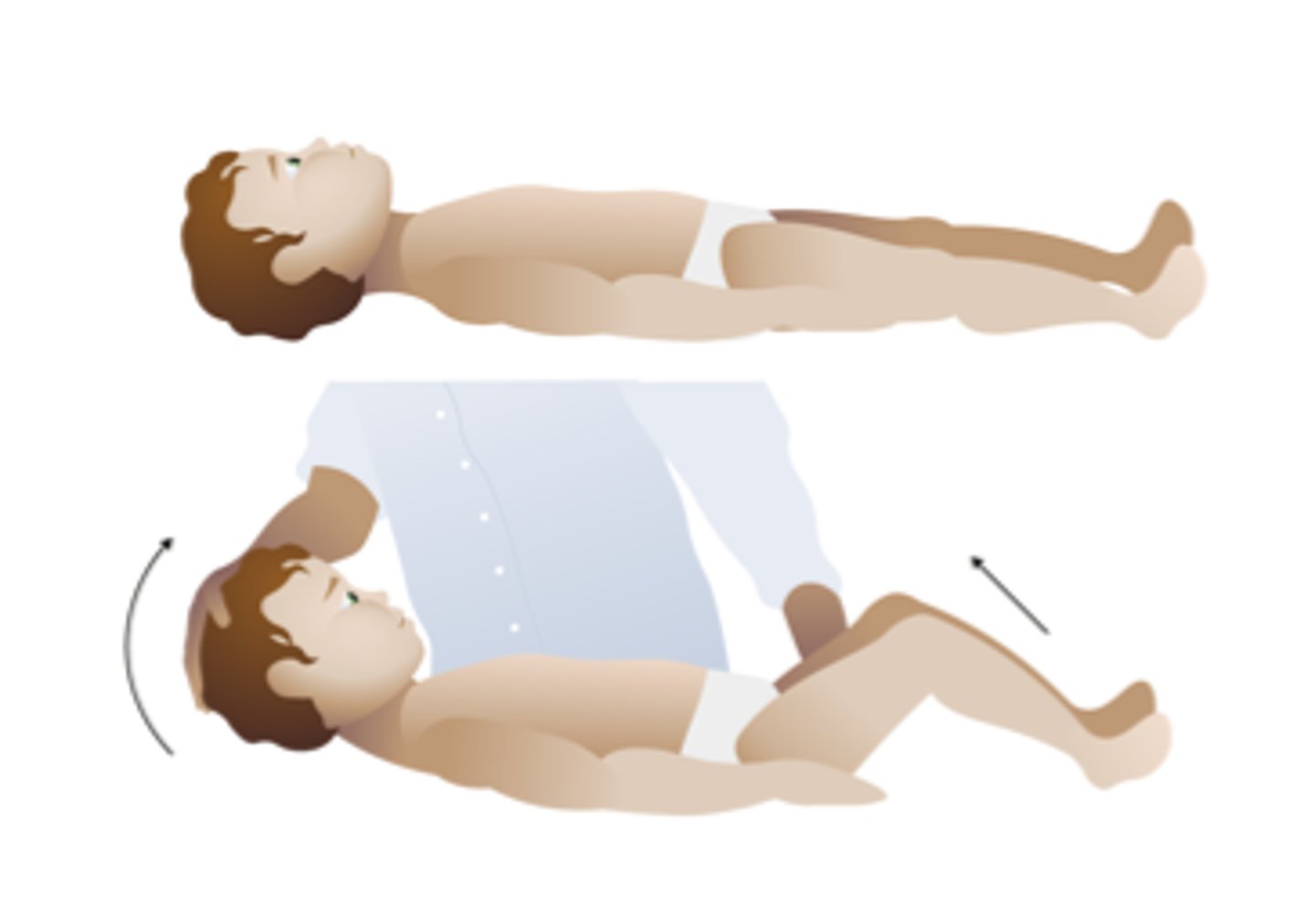
Brudinski's sign
test for meningitis
Severe neck stiffness causes a patient's hips and knees to flex when the neck is flexed.
name some complications of meningitis
hearing loss
memory problems
learning disabilities
brain damage
bone and joint problems
amputation
trouble walking
recurrent seizures
kidney failure
shock
death
if meningitis is suspected, what is the CSF tested for from a LP?
WBC count
gram stain to identify bacteria
glucose (low in meningitis)
protein (high ")
lactate
is neisseria meningitidis gram positive or negative?
negative. it is diplococci
treatments for bacterial meningitis
antibiotic
steroids - eases brain swelling
fluids - prevent dehydration
oxygen - if trouble breathing
symptoms of meningitis
headache, fever, nuchal rigidity
photophobia
phonophobia
altered mental state
seizures
what level of the spinal cord is a lumbar puncture performed?
subarachnoid space at the L3-4 or L4-5 interspace
what colour is healthy csf from a LP?
colourless / clear - may also appear so in VIRAL meningitis
risk factors for meningitis in neonates
low birth weight (<2500 g)
premature birth (<37 weeks)
premature rupture of membranes (before the onset of labor or regular contractions)
septical or traumatic birth
fetal hypoxia
maternal peripartum infection
risk factors for meningitis in children and adults
unvaccinated
age (viral most common in <5yrs, bacterial <20yrs old).
pregnancy
weakened immune system
sneezing, coughing, kissing
season
smoking
at what age do you receive the MenACWY vaccine?
14
how can anti-bacterial agents be classified?
bacteriostatic or bactericidal
chemical structure
target
what are some targets of antibiotics?
cell wall synthesis
cytoplasmic membrane
cell metabolites
protein synthesis (transcription or translation)
inhibit nucleic acid synthesis (DNA or RNA)
name two beta-lactams
penicillin
cephalosporins
what time of bacteria are glycoprotein antibiotics effective in destroying?
gram positive ONLY
can not cross the cell wall/membranes of gram negative bacteria
name both a bacteriostatic and bactericidal class of antibiotics that inhibit 30s ribosomes
bacteriostatic - tetracyclines (e.g. oxytetracyline)
bactericidal - aminoglycosides (e.g. gentamycin)
name a class of antibiotics that inhibit DNA replication
quinolones (e.g. ciprofloxacin)
name a class of antibiotics that blocks mRNA synthesis
rifamycins (e.g. rifampicin)
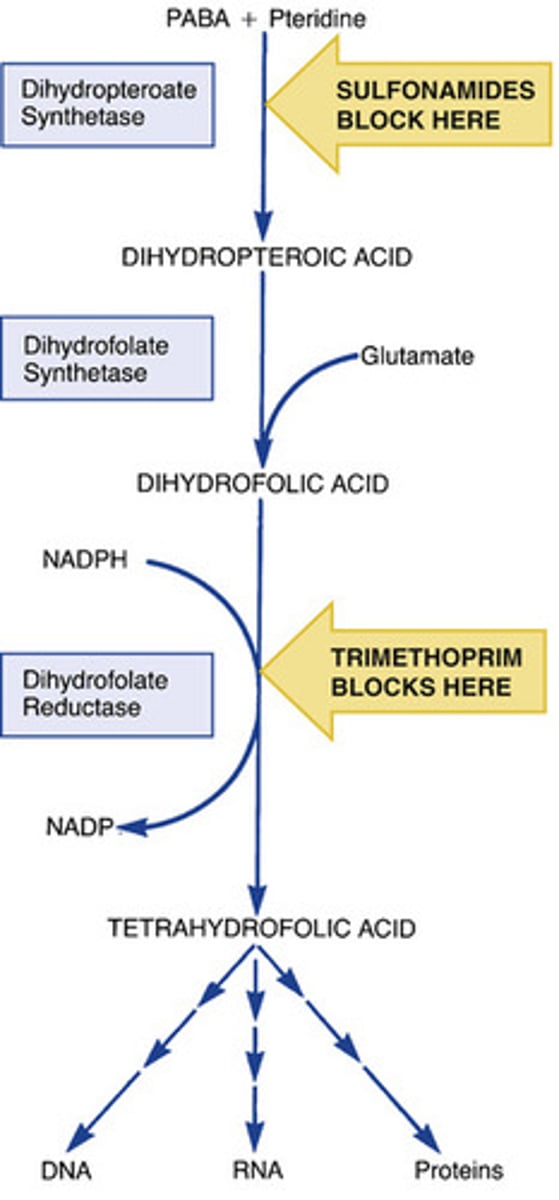
name a class of antibiotic that inhibits synthesis of nucleic acid precursors
sulfonamides (e.g. sulfamethoxazole)
trimethoprim
name a class of antibiotics that affect the cytoplasmic membrane function
polymyxins (e.g. colistin)
acts like a detergent on the membrane
give examples of narrow spectrum antibiotic classe
older penicillins (penG aka benzylpenicillin)
macrolides
vancomycin
give examples of broad spectrum antibiotic classes
aminoglycosides
2nd and 3rd generation cephalosporins
quinolones
some synthetic penicillins (e.g. amoxicillin)
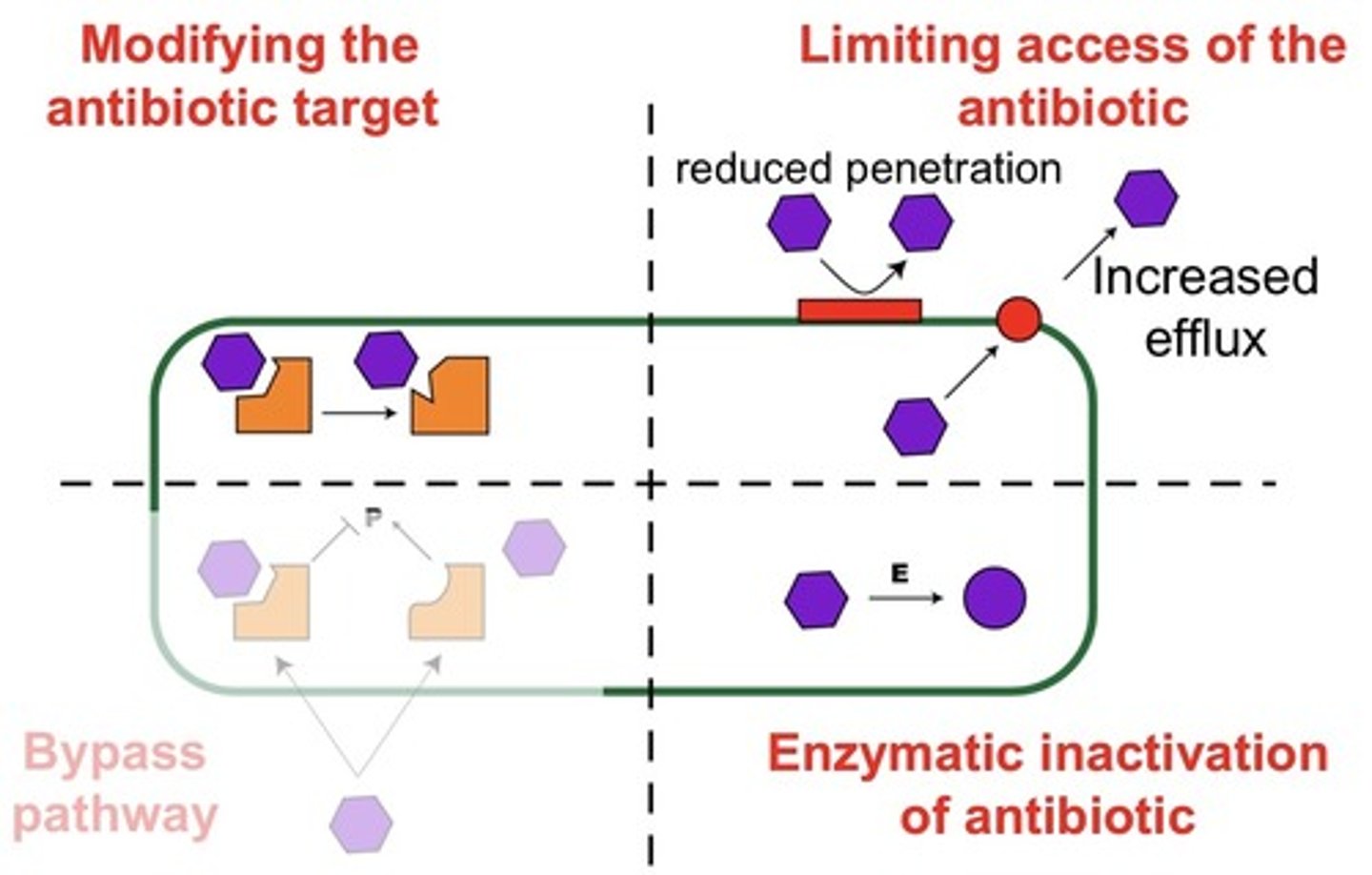
name three main methods of antibiotic resistance
1. modifying the target of the antibiotic.
2. limiting access of the antibiotic. this can either by preventing entry into the bacterium or by getting rid of it asap.
3. inactivation of the antibiotic by enzymes.
how is antibiotic resistance acquired?
1. horizontal gene transfer - acquisition of genes with resistance.
2. mutations
resistance that isn't acquired is innate e.g. bc the bacterium is gram positive so gram negative antibiotics don't work.
how is antibiotic resistance being tackled?
- Education on antibiotic use, clinically and for the public.
- better stewardship of antibiotics
- Extending life of existing antibiotics.
- Potentiate activity for existing drugs.
- Good practices in the clinic
- Facilitated by surveillance (e.g. global AMR surveillancesystem (GLASS); research funding
what are the key differences in the flagella of eukaryotic and prokaryotic cells?
- Prokaryotic flagella are smaller and simple in structure, whereas eukaryotic flagella are larger and complex in structure.
• Prokaryotic flagella are made up of flagellin protein while eukaryotic flagella are made up of tubulin.
• The movement of prokaryotic flagella is proton driven, whereas the movement of eukaryotic flagella is ATP driven.
• Prokaryotic flagella have rotator movement, whereas eukaryotic flagella have blending movement.
• Unlike the prokaryotic flagella, eukaryotic flagella have 9+2 arrangement of microtubules.
• Prokaryotic flagella are located outside of the plasma membrane, whereas the flagella in eukaryotes are covered with the plasma membrane.
List four commonly used techniques to identify and classify bacteria associated with infection and briefly describe the methods used.
PCR
gram staining
acid fast staining
biochem (testing for presence of an enzyme)
selective testing (using a medium that only allows one type of bacteria to populate or adding chemicals that cause this effect).
ADD ANS FROM 3/11/23 FROM OLU
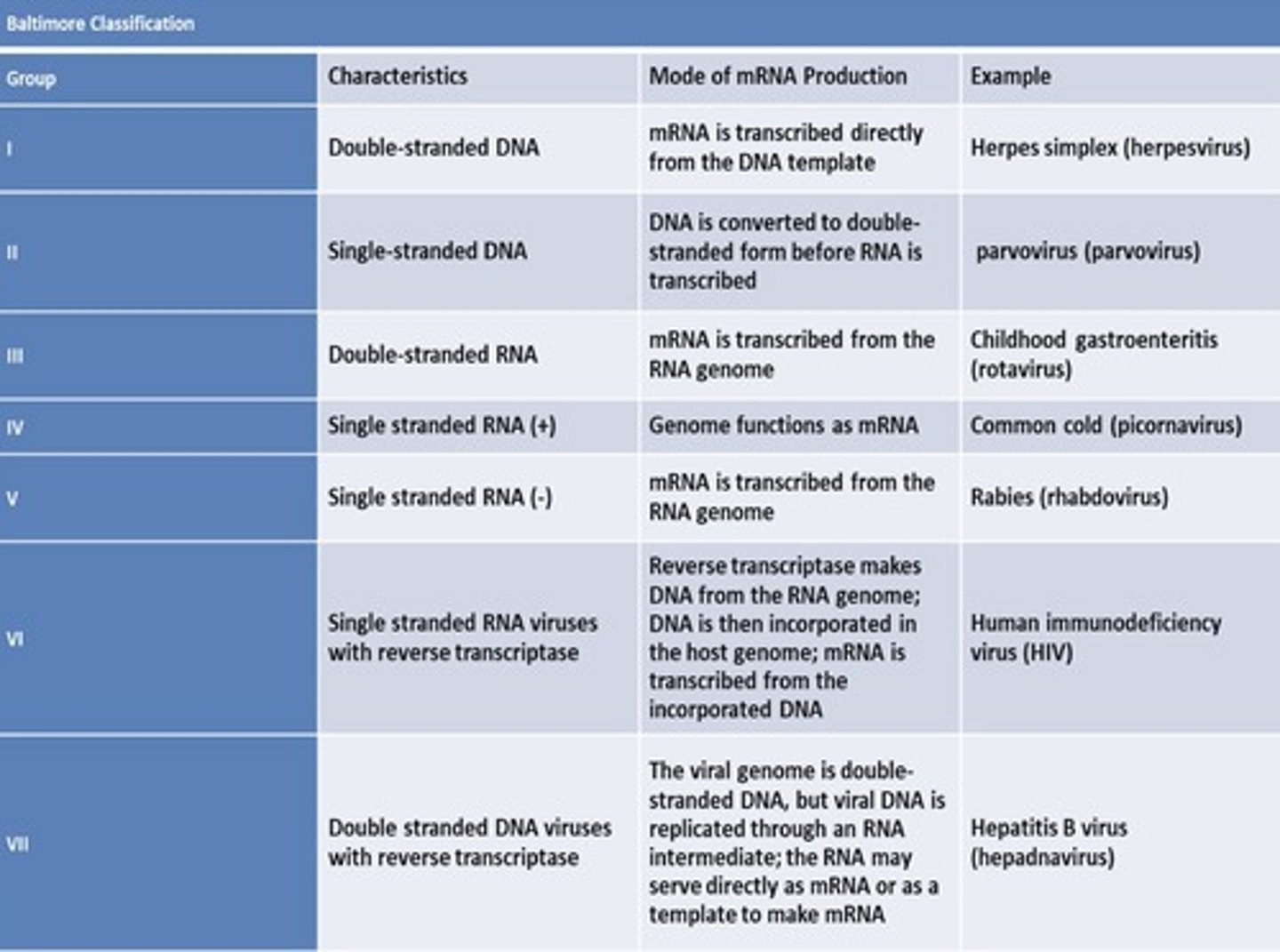
outline the baltimore viral classification