MCAT Gen Chem Review - Ch. 3 + 4: Bonding and Chemical Interactions; Compounds and Stoichiometry
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Bonding
Molecules
Chemical bonds
Octet rule
Incomplete octet
Expanded octet
Odd numbers of electrons
Types of Bonds
Ionic Bonding
Covalent bonding (coordinate covalent)
Ionic bonds
Cation
Anion
Crystalline lattice
Covalent bonds
Single/Double/Triple covalent bond
Bond order
Bond length
Bond energy
Polarity
Nonpolar covalent bond (electronegativity difference <0.5)
Polar covalent bond
Polar covalent bond (electronegativity difference 0.5-1.7)
Partial negative/positive charge
Dipole moment (p = qd) (vector quantity)
Debye units (Coulomb meters)
Covalent Bond Notation
Coordinate covalent bond
Bonding electrons
Nonbonding electrons
Lewis structure
Lewis dot diagrams
Formal charge
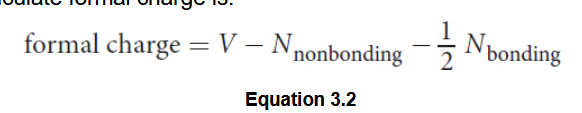
Resonance
Resonance structure
Resonance hybrid
Geometry and Polarity
Valence shell electron pair repulsion (VSEPR) theory
Electronic geometry
Molecular geometry
Coordination number
Ideal bond angle
Atomic and Molecular Orbitals
Molecular orbital
Bonding orbital
Antibonding orbital
Sigma bond
Pi bond
Intermolecular forces
London dispersion forces/van der Waals force
Dipole-dipole interactions
Hydrogen bonds (FON)
Molecular weight + Mole
Molecule
Formula unit
Formula weight
Mole
Avogadro’s number (NA)
Molar mass
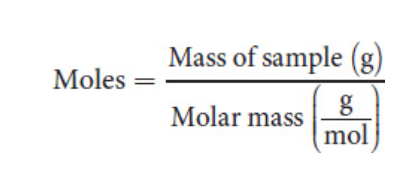
Equivalent weight
Equivalents
Gram equivalent weight (Mass of compound / gram equivalent weight)
Normality (N) (Normality / n)
Molarity
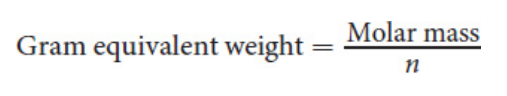
Representation of Compounds
Structural formulas
Law of Constant Composition
Empirical formula
Molecular formula
Percent composition
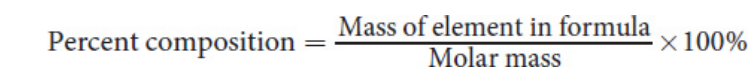
Types of Chemical Reactions
Combination reaction
Decomposition reaction
Combustion reaction
Single-displacement reaction
Double-displacement reaction
Neutralization reactions (+ salt)
Stoichiometry
Conservation of mass
Conservation of charge
Stoichiometric coefficients
Limiting reagent
Limiting reagent
Excess reagents
Yield
Theoretical yield
Actual yield
Percent yield

Cations + Anions
Ionic bonds
Oxidation states
Ionicity
Electrolytes
Solvate