IMED1003 - Energy Stores (L9)
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Energy metabolism - generating ATP
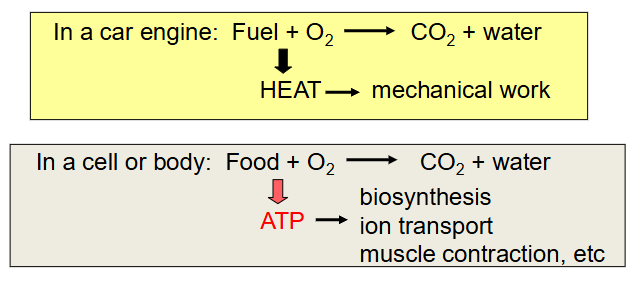
ATP levels must be maintained or the cell runs down and dies very quickly - this is what happens when we are deprived of oxygen
- ATP is made by burning fuels
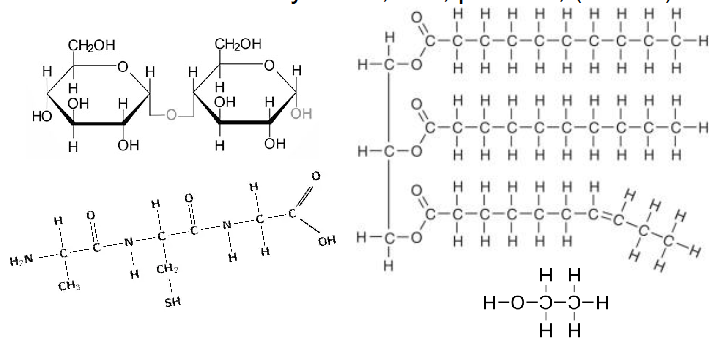
Reduced Bonds
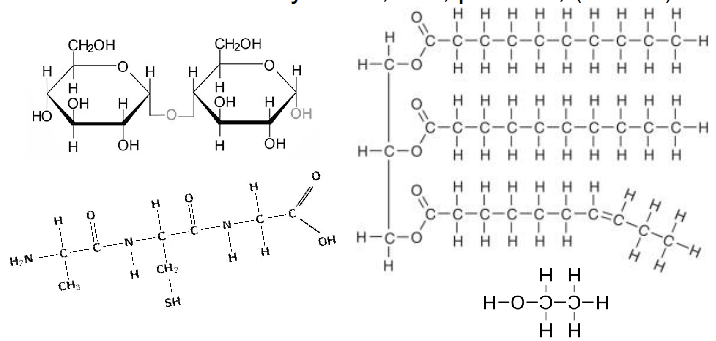
- Main fuels = carbohydrates, fats, proteins, (alcohol)
- These contain lots of reduced bonds
- electrons are not shared with Oxygen (C-H, C-C or C-N)
- oxidised bonds are gain of bonds to more electronegative atoms or loss of bonds to less electronegative atoms like hydrogen (signifies oxidation) (this increases oxidation state of carbon atom in question)
Oxidation and Reduction
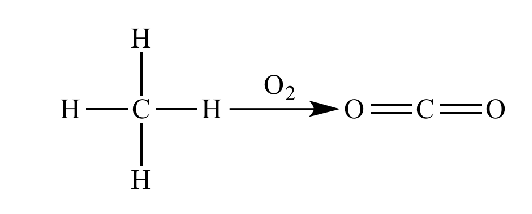
- during catabolism, electrons are removed to become part of a bond with O
- This is oxidative metabolism - we need O2 to make enough ATP in our cells
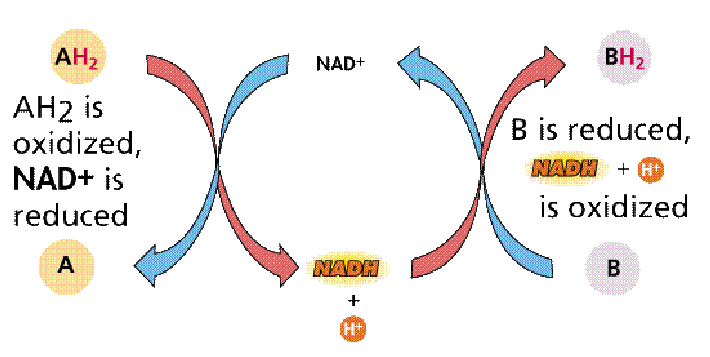
Redox Reactions - Dehydrogenase
- Redox: Can involve simply electorn transfer or involve transfer of H (as in NADH)
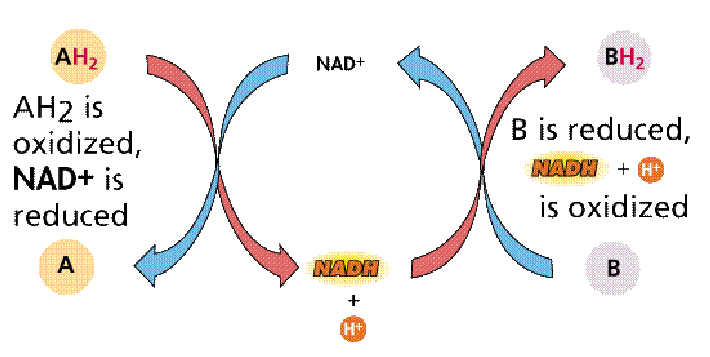
Energy Storage in Cells
- Oxidation of foods releases energy, which is stored in other molecules that are used to perform work
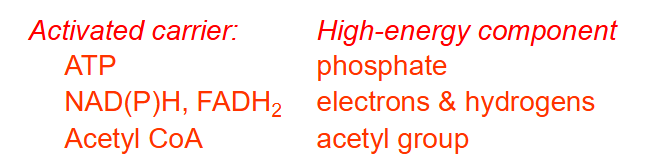
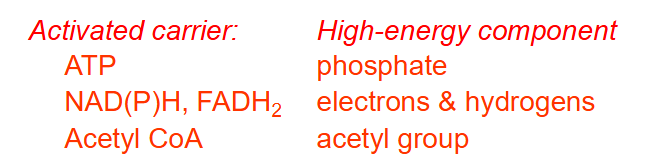
The main high energy compounds in a cell are:
ACTIVATED CARRIER AND HIGH ENERGY COMPONENT:
- ATP: phosphate
- NAD(P)H, FADH2: electrons and hydrogens
- Acetyl CoA: acetyl group
- energy can also be stored as ion gradients and in other high energy phosphate bonds (e.g PPi, pyrophosphate) DNA Replication)
- energy can be transferred between these
NAD(P)H
refers to NADH or NADPH (different things)
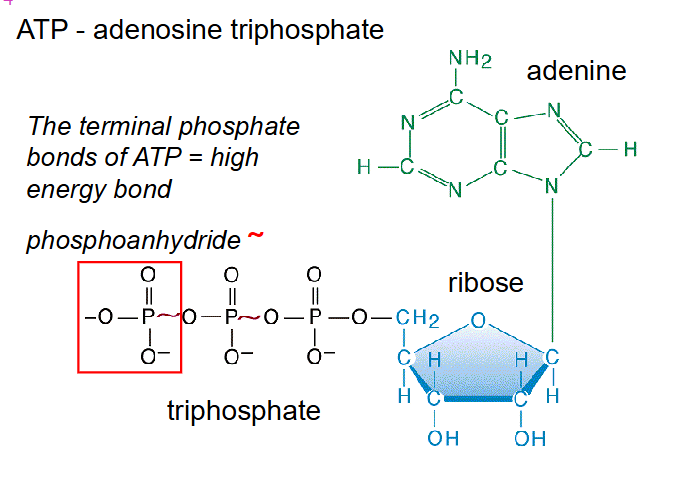
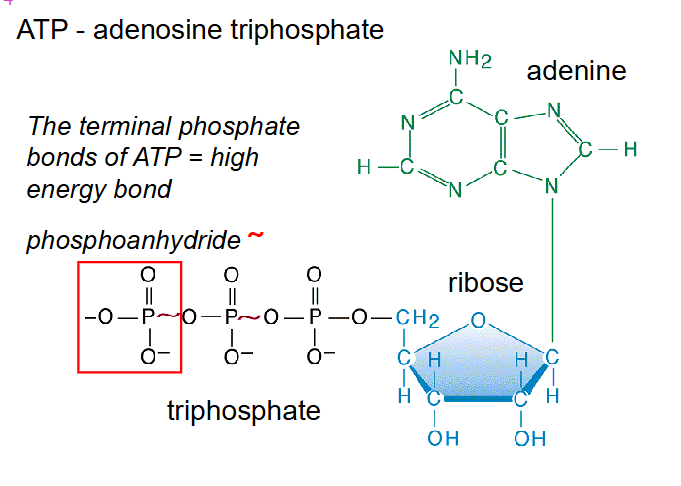
ATP - adenosine triphosphate
- The terminal phosphate bonds of ATP = high energy bond
- phosphate bonds are referred to phosphoanhydride bonds (links two phosphates together)
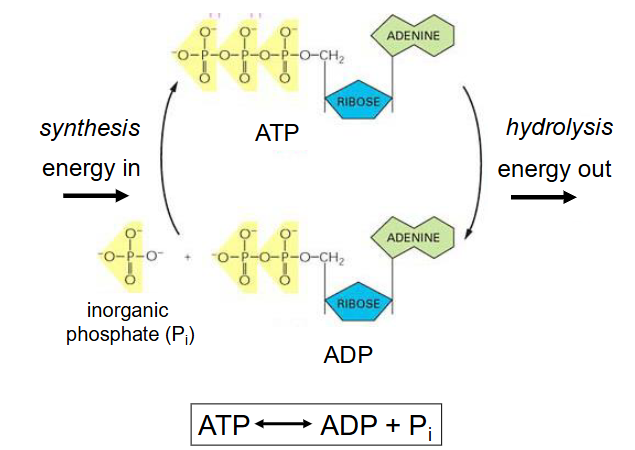
ATP = energy currency
ATP <-> ADP + Pi (inorganic phosphate)
- synthesis of ATP from ADP is endothermic (energy out)
- hydrolysis of ATP to ADP is exothermic (energy out)
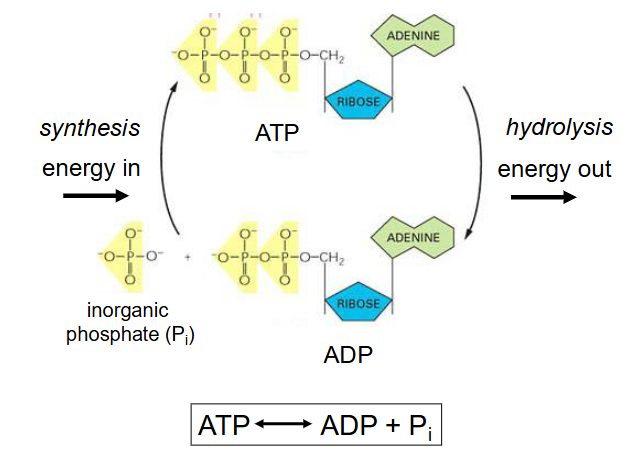
ATP Hydrolysis
- has -ve change in G
- yields 29.3kJ/mol of energy
- This energy can be used to drive other reactions such as formation of new bonds and molecules
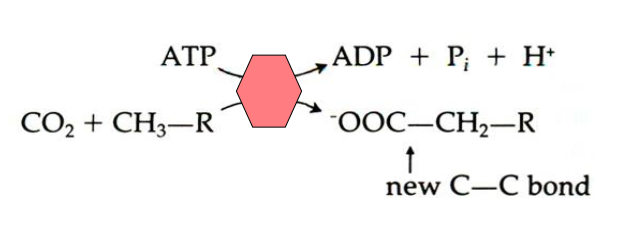
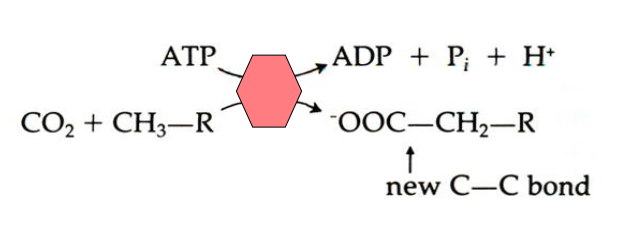
- Energetically unfavourable reaction driven by ATP Hydrolysis
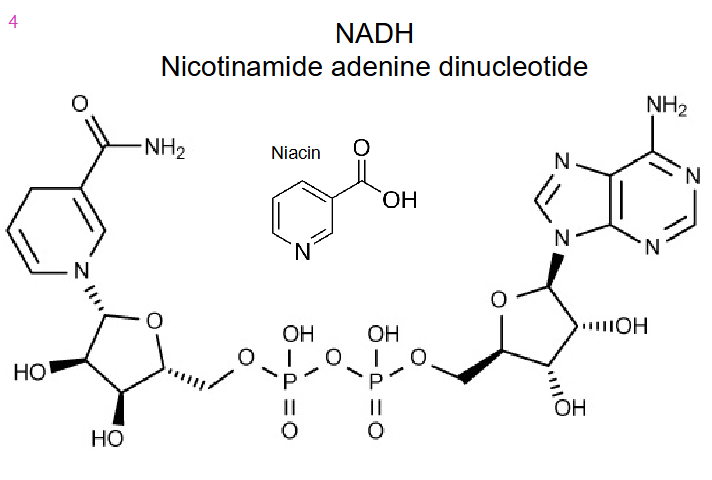
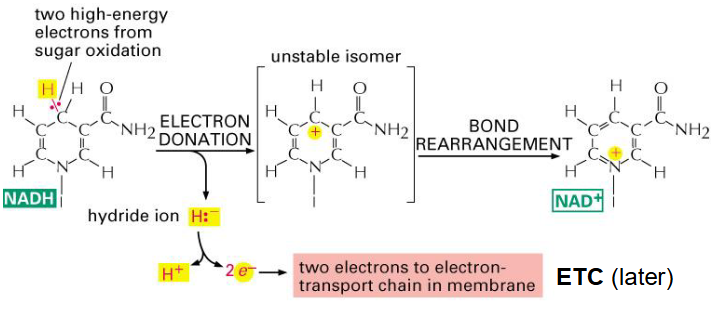
Structure of NADH
- Nicotinamide adenine dinucleotide
- niacin B3 is the precusror to make NADH by adding NH3
- source of Niacin B3 is various foods in diet
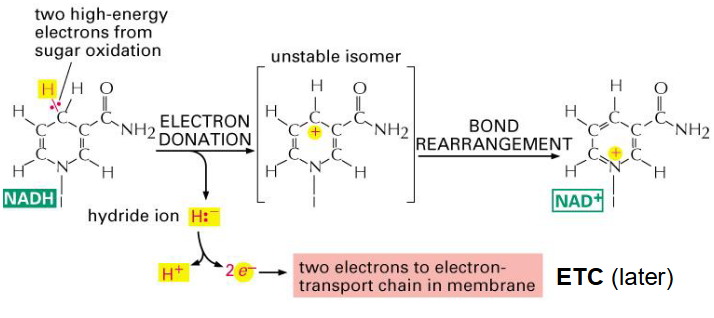
NADH
- its an important electron carrier
- Cellular currency of reductive potential energy produced during respiration
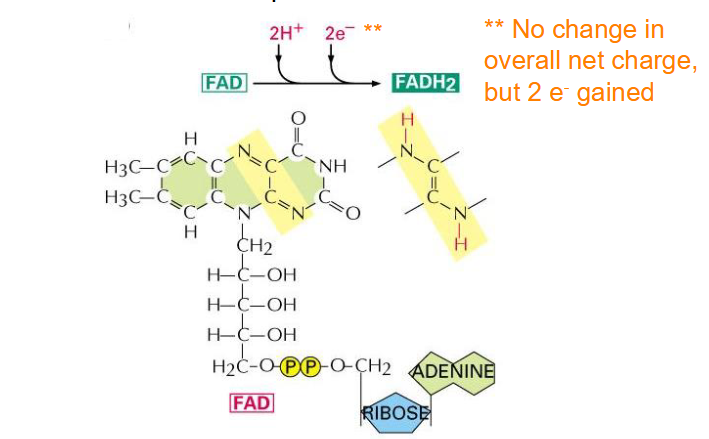
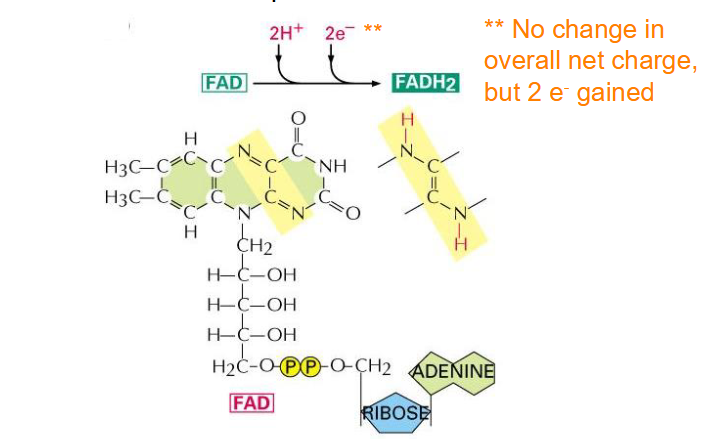
Structure of FADH2
- Flavin adenine dinucleotide
- another important electron carrier
- not change in overall net charge but 2 e- gained
- capable of undergoing reversible oxidation and reduction reactions, which is one of the reasons its an ideal electron carrier
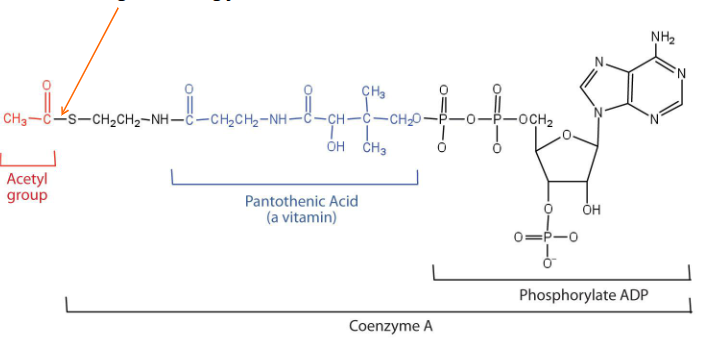
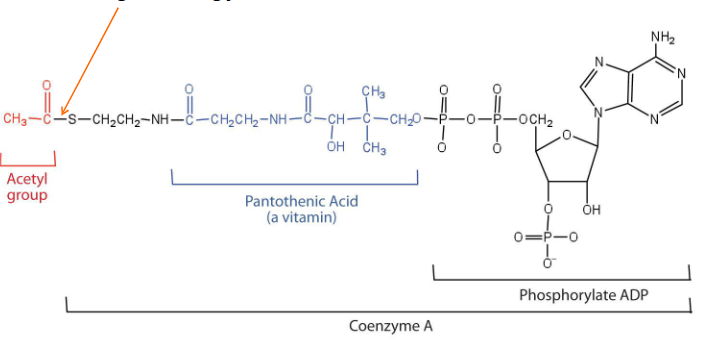
Acetyl Coenzyme A
- also known as Acetyl CoA
- is used to add 2C units to other molecules
- Has high energy thioester bond that facilitates this
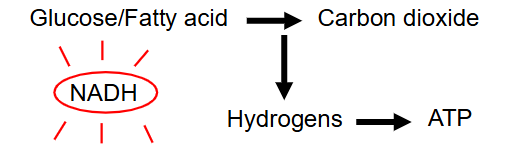
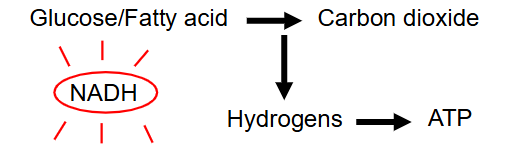
Oxidation of Fuels (Respiration)
MAJOR STORES:
- Carbohydrate
- Glycogen/Glucose in Animals
- Starch/Sucrose in Plants
- Fats (triglycerides)
- Alcohol (not!)
- hydrogens are stripped out of the fuels
- Multi-step process
- As H is removed, the fuels gradually broken down to CO2 (have oxidised the reduced bonds)
- remember that removal of H means oxidation
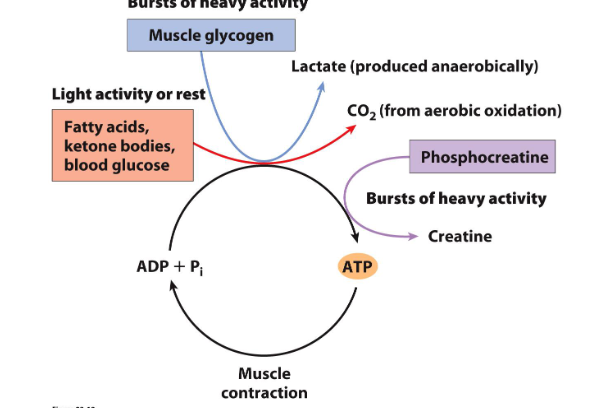
Phosphagens: high energy compounds for bursts of activity in muscles
- important for energy transfer and storage
- high energy compounds
- these are important when a burst of activity is required such as muscles
- when demands for ATP is high we use these
- e.g muscle glycogen is one example of these stores of energy that can be utilised during heavy activity
- other is phosphocreatine
- during bursts of heavy activity, both muscle glycogen and phosphocreatine can be broken down to generate either lactate or creatine in the case of phosphocreatine
- provides an alternate source of energy when demands are very high or in particular tissue types where there is high demand for energy at particular times
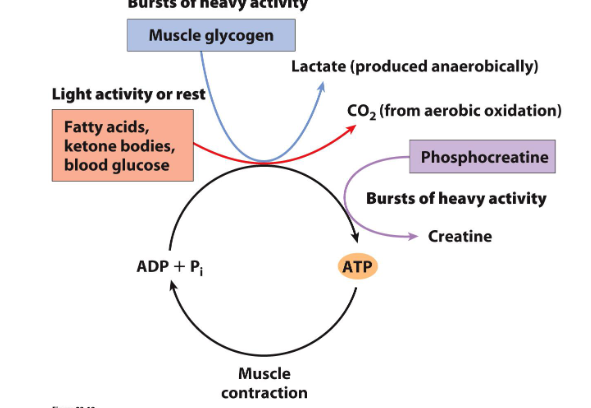
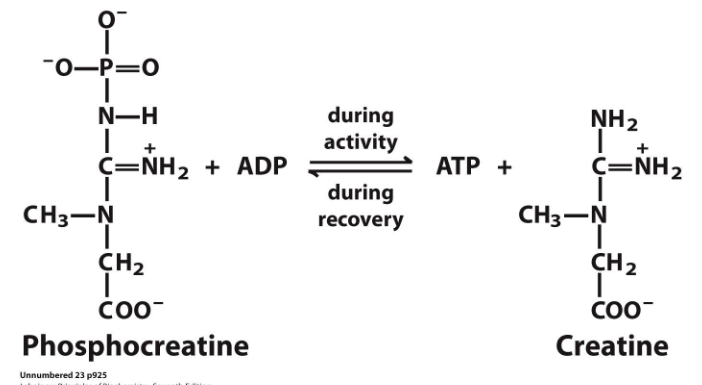
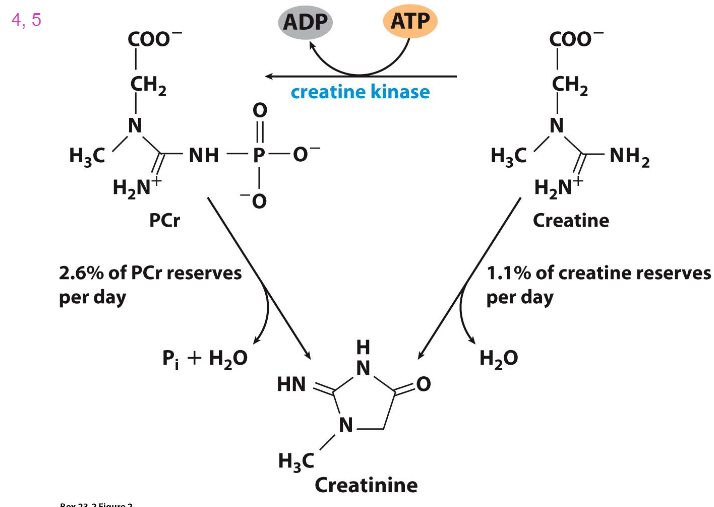
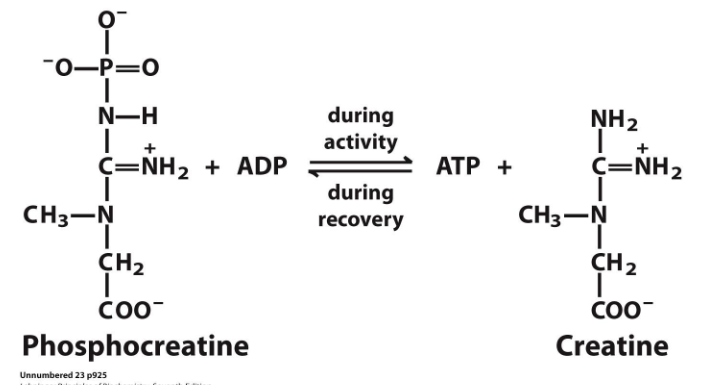
Phosphocreatine buffers the ATP pool during bursts of activity
- phosphocreatine reacts with ADP during activity to produce ATP and creatine. (this makes sense since it loses a phosphate group and that phosphate group combines with ADP to make ATP
- during recovery ATP reacts with creatine to form phosphocreatine and ADP
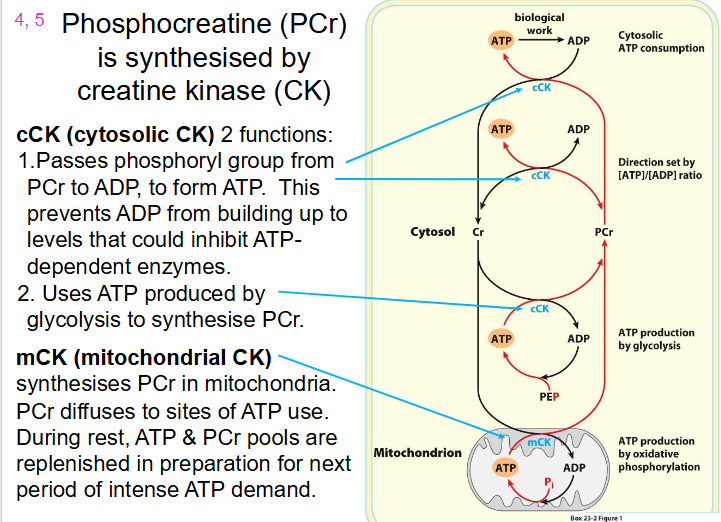
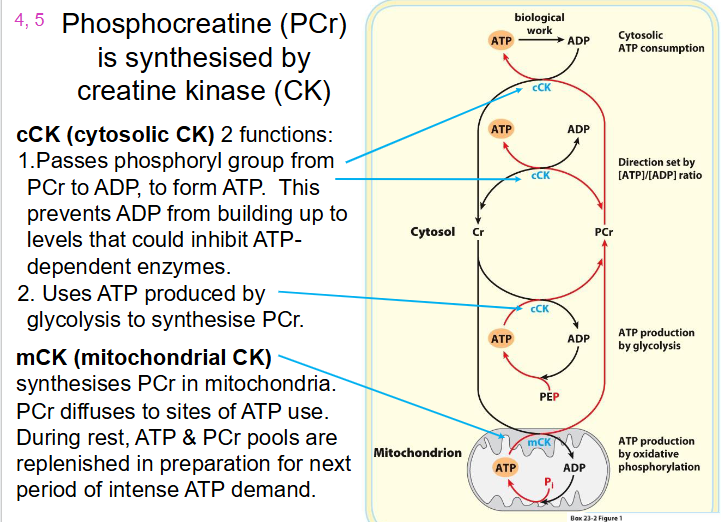
How Phosphocreatine (PCr) is synthesised
- Synthesised by Creatine Kinase (CK)
cCK (cytosolic CK) has 2 functions:
1. Passes phosphoryl group from PCr to ADP, to form ATP. This prevents ADP from building up to levels that could inhibit ATP dependent enzymes
2. Uses ATP produced by glycolysis to synthesise PCr
mCK (mitochondrial CK):
- synthesises PCr in mitochondria
- PCr diffuses to sites of ATP use
- during rest, ATP and PCr pools are replenished in preparation for next period of intense ATP demand
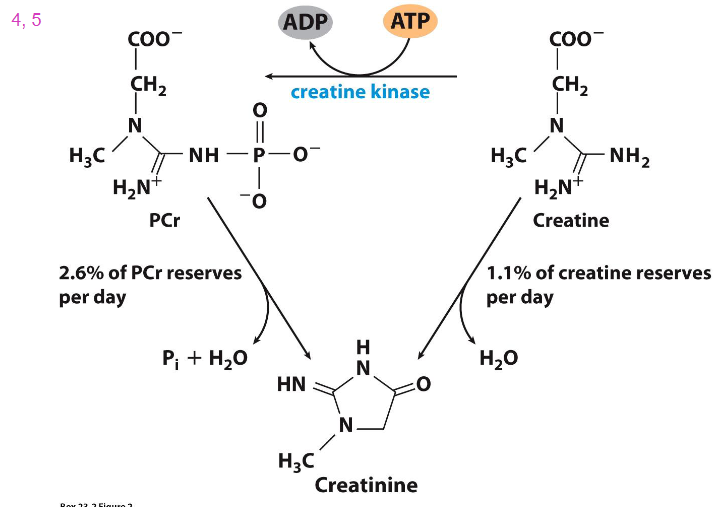
Closer Look at Chemical Reaction for CK (creatine kinase)
- if you dont eat meat and dairy, you have to synthesise all the creatine that you need
- if you eat meat and eggs, dairy, you have this in your diet
- we have creatine reserves