Introduction, Scope of Biochemistry, Water, Hydrogen Bonds, Buffers
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What are the levels of organization from atoms to organs?
Atoms -> Molecules -> Macro Molecules -> Organelles -> Cells -> Tissues -> Organs -> Organism
Where are all the places life on Earth exists?
Everywhere. Extremophiles are found in extreme conditions. Extremophiles are mostly Archaea.
How do you work with multiple pKa values?
As pKa rises, molecule is becoming more negative. The isoelectric point is the mid point between the +1/0 pKa and the 0/-1 pKa
if pH < pKa by one or more pH units, is the proton ON or OFF
Proton is ON
If pH > pKa by one or more pH units is the proton ON or OFF ?
Proton is OFF.
How do you calculate the pKa given the Ka?
pKa = -log10(Ka)
Formic acid has a pka of 3.75, and Acetic acid a pKa of 4.76. Which is the stronger acid ?
Low pKa = stronger Weak Acid. Formic acid is the stronger of the two weak acids listed.
What is the pKa of HCl ?
Strong acids, such as combinations of a halide and hydrogen, essentially have no pKa, because they tend to fully dissociate in water
What is the Henderson-Hasselbalch equation?
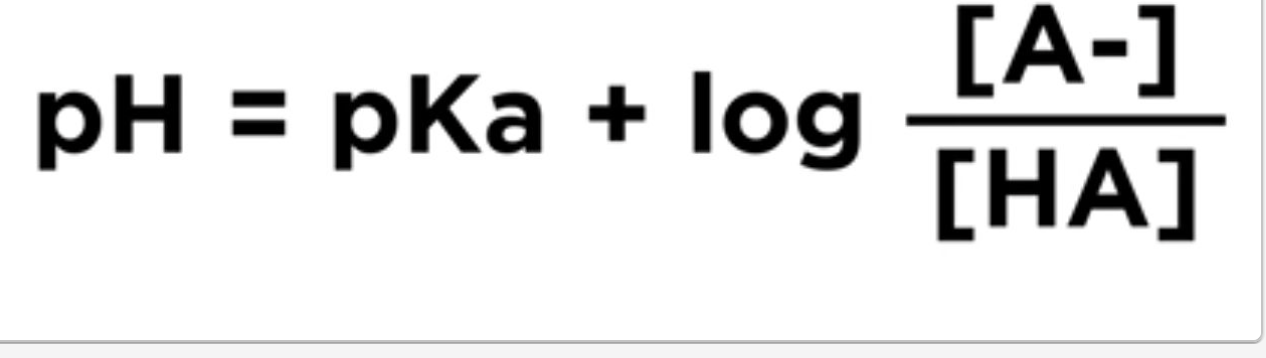
What is a buffering Region?
A "buffering region" in chemistry refers to a specific range on a titration curve where the pH of a solution remains relatively constant, usually occurring when a weak acid is titrated with a strong base, and it's the section where the solution effectively resists significant pH changes due to the presence of a buffer system; essentially, it's the "flat" part of the curve where the pH stays stable despite the addition of small amounts of acid or base
How it works:
A buffer solution contains a weak acid and its conjugate base, which can neutralize added hydrogen ions (H+) or hydroxide ions (OH-) to maintain the pH.
Midpoint of the buffer region:
This point occurs when exactly half of the weak acid has reacted, resulting in equal concentrations of the acid and its conjugate base, where the pH is equal to the pKa of the acid.
How is Acid dissociation graphed?
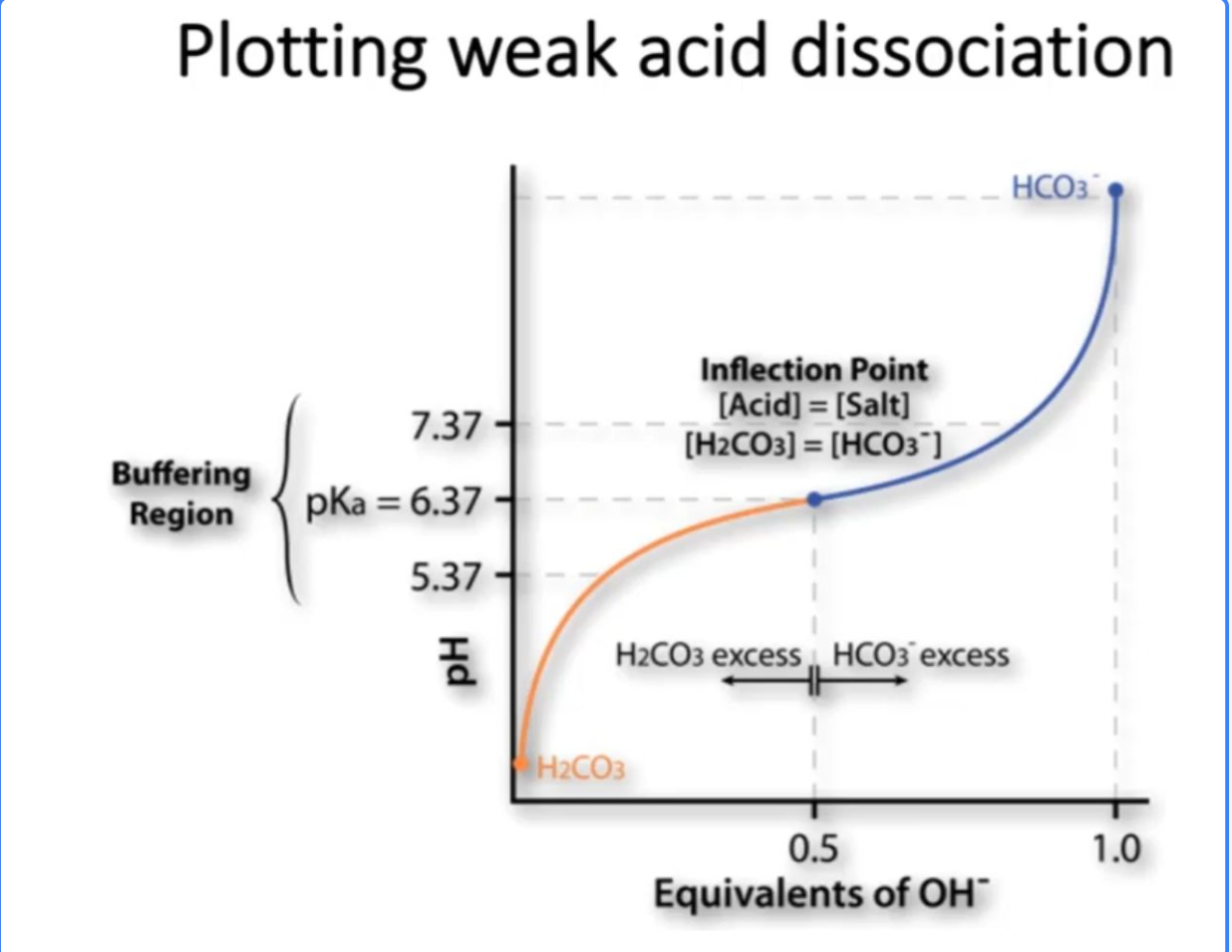
How do we describe acid dissociation?
HA <--/--> [H+] + [A-] . The Acid HA has one more proton than its Salt A-
What is pH?
pH = -Log[H+]
Give Examples of weak and strong acids
Strong acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3), perchloric acid (HClO4), hydrobromic acid (HBr), and hydroiodic acid (HI); while weak acids include acetic acid (CH3COOH), carbonic acid (H2CO3), citric acid, oxalic acid, phosphoric acid, and formic acid (HCOOH)
What is pKa?
pKa is a measurement of a molecule's acidity or basicity, and how likely it is to keep a proton at its ionization center. It's calculated as the negative base-10 logarithm of the acid dissociation constant (Ka)
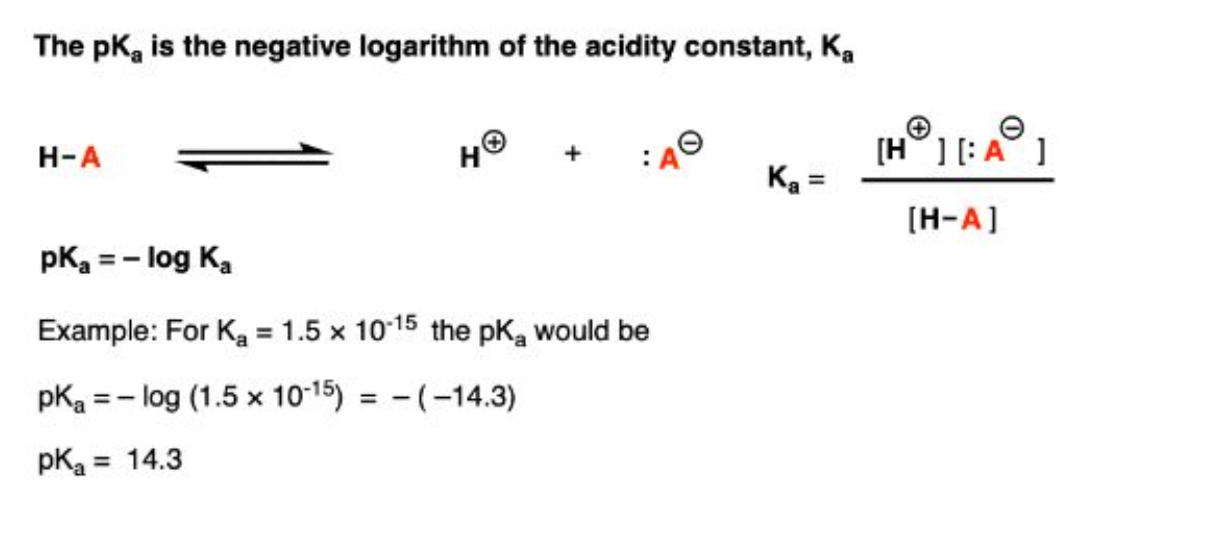
How is hydrophobicity useful for protein structure?
Hydrophobicity is crucial for protein structure because it acts as a major driving force in protein folding, causing hydrophobic amino acids to cluster together and bury themselves inside the protein core, away from the aqueous environment, thus stabilizing the folded structure and contributing significantly to its overall stability; this phenomenon is known as the "hydrophobic effect."
Contrast a micelle with a liposome. How does a liposome work to carry drugs?
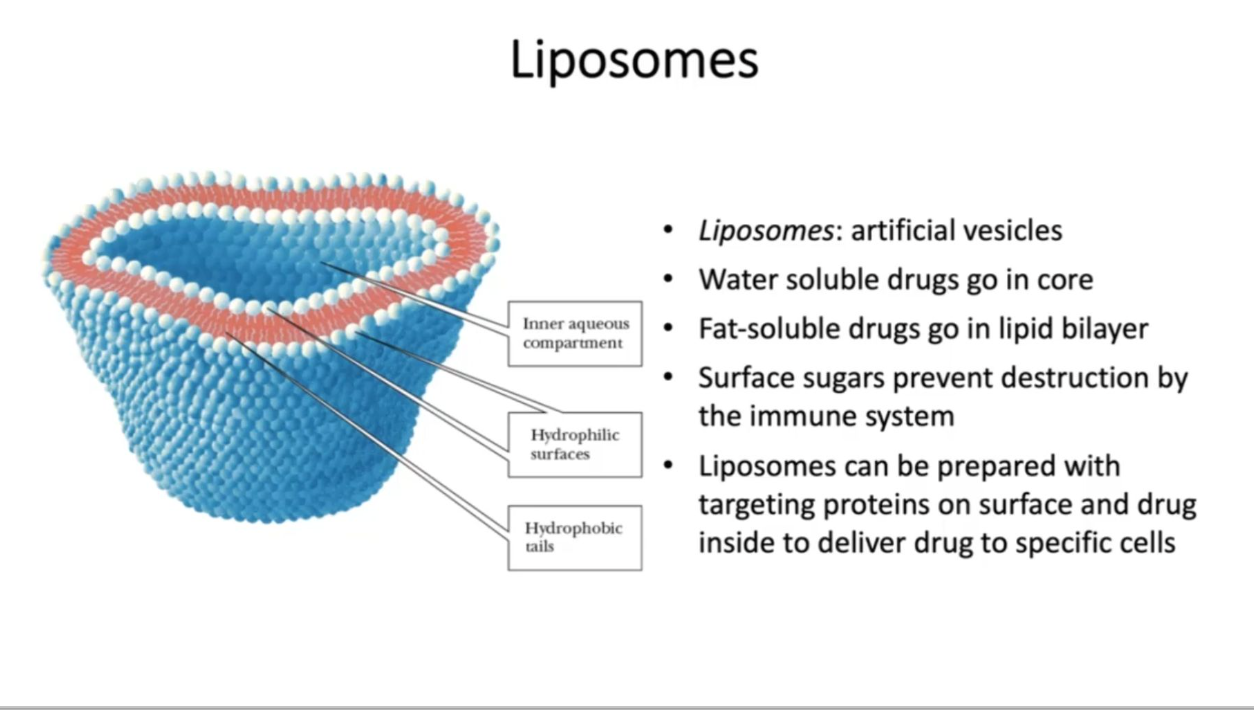
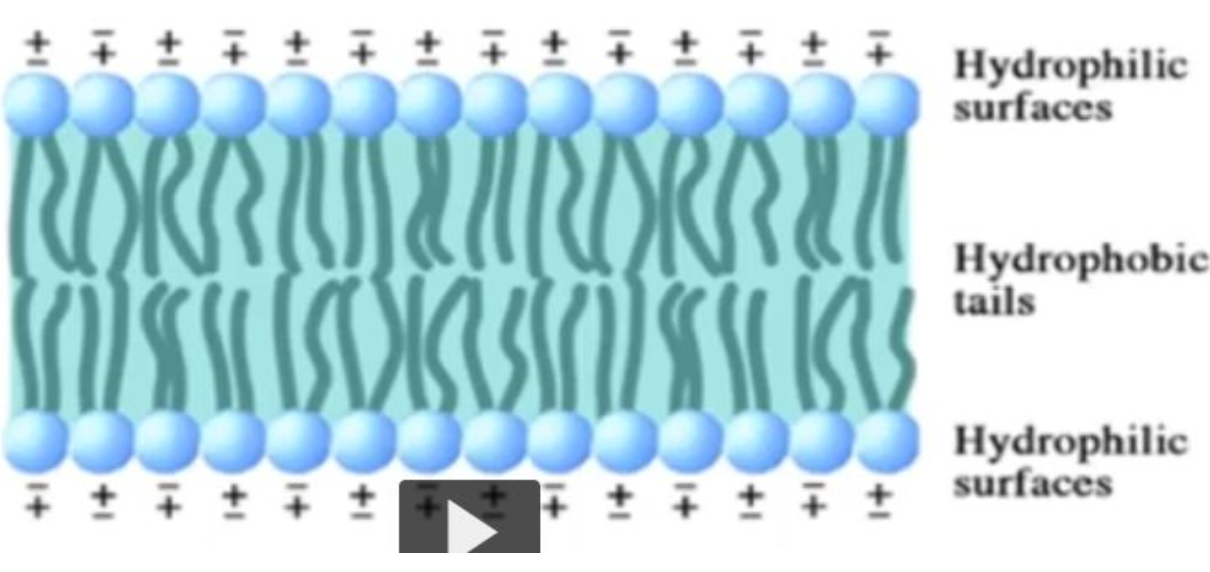
What is a biological membrane made from? How is it structured?
A phospholipid bilayer is a double layer of phospholipid molecules that form the basis of cell membranes, where the hydrophilic (water-loving) phosphate heads face outward towards the aqueous environment, while the hydrophobic (water-hating) fatty acid tails are oriented inwards, creating a barrier between the cell interior and exterior; essentially acting as a stable, fluid barrier that separates aqueous compartments within a cell.
What is micelle? How does soap work?
Micelle: A spherical (clustered) arrangement of amphipathic molecules in water. Hydrophobic parts cluster away from water forming an inner core, hydrophillic parts face outward to interact with water. Soap micelles trap non-water soluble oils and grime in its hydrophobic core.
Define amphiphatic/amphiphilic? Give an example
An amphiphatic/amphiphilic molecule has both a hydrophilic surface and a hydrophobic surface. A good example is a phospholipid - the polar head is hydrophilic, while the non-polar tail is hydrophoblic, leading to the natural formation of a bilayer in which the nonpolar tails form the inner part of the cellular membrane and the polar heads form the two surfaces of the membrane, one interacting with the aqueous extracellular enviroment and the other interacting with the aqueous cytoplasm.
What do hydrophilic and hydrophobic mean? Give some example molecules for each.
Hydrophilic molecules, such as polar amino acids and sugars, dissolve readily in water. Hydrophobic molecules, such as non-polar amino acids and fats, do not dissolve in water (Immiscible with water).
What is Gibbs Free Energy?
Gibbs free energy, denoted G, combines enthalpy and entropy into a single value. The change in free energy, ΔG, is equal to the sum of the enthalpy plus the product of the temperature and entropy of the system(ΔG =ΔH - TΔS). ΔG can predict the direction of the chemical reaction under two conditions:
constant temperature and
constant pressure.
If ΔG is positive, then the reaction is nonspontaneous (i.e., an the input of external energy is necessary for the reaction to occur) and if it is negative, then it is spontaneous (occurs without external energy input). If it is zero, the reaction is at equilibrium.
Define Oxidation and Reduction
Oxidation is defined in terms of the loss of electrons, gain of oxygen, loss of hydrogen, or an increase in oxidation number. Reduction is defined in terms of gain of electrons, loss of oxygen, gain of hydrogen, or a decrease in oxidation number.
Where in our bodies are hydrogen bonds found?
Hydrogen bonds are primarily found in the DNA molecule within the human body, where they hold the two strands of the double helix together by connecting complementary base pairs (like Adenine to Thymine and Cytosine to Guanine); they are also present in proteins, contributing to their structure (alpha helixes and beta sheets) and function through interactions between amino acid residues. Hydrogen bonds exist between all water molecules in the human body.
What are the main types of chemical bonds, from strongest to weakest?
ionic bonds (strongest) Example - NaCl bond is -786kJ/mol, covalent bonds (moderately strong) Example - O-H sigma bond is -460kJ/mol, hydrogen bonds (weaker) roughly 20 kJ/mol, Ion-Dipole also roughly 20 kJ/mol, and Van der Waals interactions (weakest) such as hydrophobic interactions , roughly 4 to 12 kJ/mol.
What is unique about hydrogen bonds?
Hydrogen bonds are unique because they are a special type of dipole-dipole interaction that occurs when a hydrogen atom, bonded to a highly electronegative atom like oxygen or nitrogen, is attracted to another electronegative atom with a lone pair of electrons, creating a strong intermolecular force that is significantly stronger than other dipole-dipole interactions, yet weaker than covalent bonds; this property is largely due to the small size of the hydrogen atom and the large electronegativity difference between hydrogen and the other atom it's bonded to
What is polarity?
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms.
What are the chemical properties of water which make it unique?
Water is highly polar and is di-polar, and is a hydrogen bond donor and acceptor. Water is a good solvent due to its polar nature. Due to the fact that it can hydrogen bond with itself, water has a very high spread between its melting and boiling points, leading to both a high heat capacity and a high heat of vaporization. It has both cohesive and adhesive properties and is less dense as a solid than as a liquid.
What are the electronegativities of CHONPS and what does this term mean?
Carbon - 2.5 ; Hydrogen 2.1 ; Oxygen 3.5 ; Nitrogen 3.0 ; Phosphorous 2.2; Sulfur 2.6
How many bonds can be formed from the elements C, O, N, P, S
Carbon can form 4 bonds, Oxygen two bonds, Nitrogen three bonds, Sulfur two bonds, and Phosporus 5 bonds. These are sigma bonds. Pi bonds use up two "bonding points" , so carbon can form 4 sigma, or 2 pi, or 2 sigma and 1 pi bond.
Describe the differences between Anabolism and Catabolism. What is the scientific definition of metabolism?
Anabolism requires energy, and is reductive. Catabolism releases energy and is oxidative. Anabolism takes the products of catabolism plus energy to form the four classes of macromolecules - Lipids, Polysaccharides, proteins, and Nucleic Acids. Catabolism breaks down the macromolecules into their buildings blocks; fatty acids, sugars, amino acids, and nucleotides. The products of each of these processes can serve as the substrate for the other process - anabolic products can be broken down by catabolic processes, catabolic products can be used by anabolic processes to build new macromolecules.
What are the major building blocks of macromolecules in our bodies?
Nucleic Acids are made of nucleotides ; Proteins are formed from amino acids ; Polysaccharides are chains of sugars. Lipids are formed from fatty acids.
Classify as either Prokaryote or Eukaryote 1. Humans 2. Plants 3. Bacteria 4. Yeast
Humans, Plants, and yeast are all Eukaryotes. Humans are multi-celled and yeast is single celled. Bacteria are Prokaryotes.
What is a Eukaryote? What is a prokaryote? What are the major Differences?
Prokaryotes are
a microscopic single-celled organism that has neither a distinct nucleus with a membrane nor other specialized organelles. Prokaryotes include the bacteria and cyanobacteria.
Eukaryotes are
1. An organism consisting of a cell or cells in which the genetic material is DNA in the form of chromosomes contained within a distinct nucleus.Has membrane bound organelles. Includes all living things that are not archaea and bacteria.