Protein structure and function
1/36
Earn XP
Description and Tags
xtra carrds
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
What are the monomers and polymers involved in forming proteins?
Amino acids are the monomers of proteins. When joined together, they form polypeptides (polymers), which fold into functional proteins.
What is the primary structure of a protein, and what is its significance?
The linear sequence of amino acids of the polypeptide chain joined by peptide bonds (coded for by the DNA). The primary structure will determine the interactions that can occur between amino acids and consequently all subsequent structures.
What defines the secondary structure of a protein, and what is its significance?
Folding of the polypeptide chains into repeating elements such as alpha helices, beta pleated sheets, and random coils.
caused by hydrogen bonds between amine and carboxyl groups between non-adjacent amino acids.,
Basically multiple primary structures (polypeptides) bonded together. Alpha helix provides elasticity and stretch, beta pleated sheet is not elastic but strong. Random coils are any other shapes that can form due to the polypeptide chainls.
significance: the secondary structure will help form the tertiary/quatenary structure of the protein by allowing only some interactions between amino acids, based on the shape formed.
What defines the tertiary structure of a protein, and what is its significance?
overall folding (3D shape) of the polypeptide, so now it's a protein 😛.
A result of the secondary structure undergoing further folding held together by various bonds (disulfide, ionic, and hydrogen) between the R group side chains. All proteins are 3D.
significance: the 3D shape determines the overall function of the protein
What defines the quaternary structure of a protein, and what is its significance?
Two or more polypeptide chains joined to form a functional protein. not all all proteins have a quatenary structure.
significance: the quatenary structure will determine the function of the protein
(Hint: Look for the word chains when trying to work out if it is quaternary. If it has multiple chains it’s a quaternary structure.)
What are examples of proteins with quaternary structure?
Rubisco, DNA polymerase, and RNA polymerase.

What is the function of regulatory proteins and an example?
they are hormones which regulate body processes, e.g. insulin regulates blood glucose levels.
What are fibrous proteins and examples?
Long, strand-like shape
Insoluble in water
Strong and stable
Structural function (not metabolic)
Fibrous proteins include contractile and structural proteins.
Examples: collagen, keratin, and elastin
What are globular proteins and examples?
Globular proteins are involved in metabolic processes and has a specific 3D shape.
Includes transport, catalytic, membrane, immunoglobulin, and regulatory, proteins.
compact
water-soluble
function as enzymes, hormones, antibodies, and transport molecules.

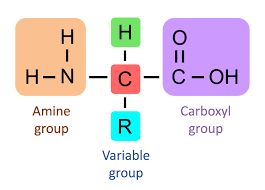
Draw the basic structure of a generalised amino acid
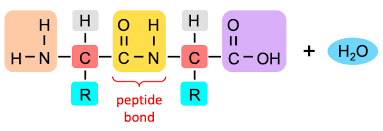
Draw the basic structure of a generalised dipeptide.
Identify how many different amino acids are present in nature and describe how they are distinctive.
There are 20 amino acids
Each amino acid has a different R/variable group.
The properties of the R groups differ e.g. Polar vs non polar, acidic vs basic etc.