core practical 2: determining enthalpy change of reaction
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
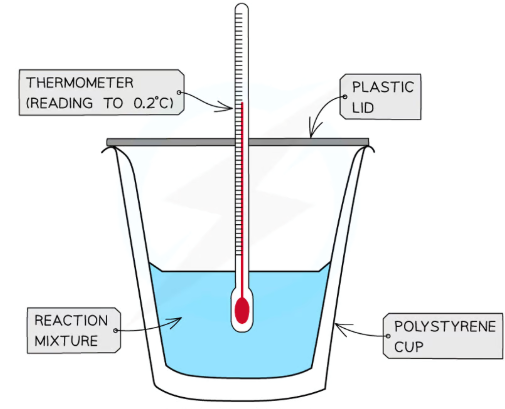
what is calorimetry?
Calorimetry is a technique used to measure changes in enthalpy of chemical reactions
A calorimeter can be made up of a polystyrene drinking cup, a vacuum flask or metal can
what is specific heat capacity?
The energy needed to raise the temperature of 1 g of a substance by 1 K
what is the principl of enthalpy changes for reaction in solution?
The principle of these calorimetry experiments is to carry out the reaction with an excess of one reagent and measure the temperature change over the course of a few minutes
sample method for a displacement reaction?
Using a measuring cylinder place 25 cm3 of the 1.0 mol dm-3 copper(II) sulphate solution into the polystyrene cup
Weigh about 6 g of zinc powder - as this is an excess there is no need to be very accurate
Draw a table to record the initial temperature and then the temperature and time every half minute up to 9.5 minutes
Put a thermometer or temperature probe in the cup, stir, and record the temperature every half minute for 2.5 minutes
At precisely 3 minutes, add the zinc powder to the cup (DO NOT RECORD THE TEMPERATURE AT 3 MINUTES)
Continue stirring and record the temperature for an additional 6 minutes
For the purposes of the calculations, some assumptions are made about the experiment:
That the specific heat capacity of the solution is the same as pure water, i.e. 4.18 J g-1 K-1
That the density of the solution is the same as pure water, i.e. 1 g cm-3
The specific heat capacity of the container is ignored
The reaction is complete
There are negligible heat losses
Why do we extrapolate graphs to find maximum temperature?
Because reaction is not instantaneous so it may lose heat to surroundings, so we don't know its true maximum temperature
The moles of which substance is used to caluculate heat transfer (Q=mcT)
moles of the limiting reactant
The steps to make a temperature correction graph are:
Take a temperature reading before adding the reactants for a few minutes to get a steady value
Add the second reactant and continue recording the temperature and time
Plot the graph and extrapolate the cooling part of the graph until you intersect the time at which the second reactant was added
enthalpy change of combustion experiment
Fill copper can with 100cm³ of water, record initial temperature with thermometer
Measure and record the mass of an empty spirit burner, add fuel and record mass
light the wick, heat water (stirring constantly) for set time/until fuel is completely burnt. Record temeprature of water. Measure mass of burner + and remaining fuel. Subtract to find mass of fuel burnt
What is Hess's Law?
The total enthalpy change of a reaction is independent of the route taken.
How do you calculate enthalpy change?
Find the heat energy released (Q=mcAt)
Find the amount of substance reacted (n=m/Mr)
Calculate the enthalpy changer per mol (changeH=Q/n)
What are errors when calculating enthalpy change?
1. Some of the heat energy is transferred to the air and
not the water.
2. Some of the heat energy produced is transferred to
the copper can and not to the water.
3. Incomplete combustion of alcohol – less heat energy
and also cause soot to form on the bottom of the
copper can.
-some alcohol evaporates
heat cappacity of beaker not taken nto account
4. The conditions are not standard. e.g. water vapour is
produced instead of liquid water.
only used mass of water not solution
If formation of water in gaseous state then value would be less exothermic/lower as energy taken in to form gas is lower
effect of using polystyrene cup instead of glass beaker
minimum temperature would be lower (1)
temperature would increase at a slower rate (> 90 s) (1)
less heat (from the surroundings) would enter the solution
if glass beaker usedThe heat loss will be greater Or • (Because) polystyrene is a better insulator Or • More energy is used to heat the container/ glass Or • (Because) the polystyrene cup has a low heat capacity
ways to increase accuracy of enthalpy of combustion
1. Put the lid on – to reduce heat loss and prevent evaporation of water
2. Use draught shield – to reduce heat loss
3. Reweigh spirit burner immediately – to reduce evaporation of
methanol
4. Use a copper calorimeter - to improve heat conduction to the water or copper oil to conduct heat from emissions
putting insulation around cup
why value mroe exothermic
-ethanol/water are not in standard stated for bond enthalpy caluclations
standard enthalpy change of combustion refers to ehtanol /water as liquids but bond energies are clauclated for gases
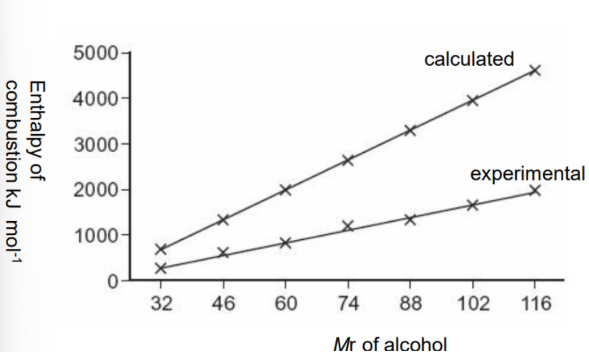
Standard enthalpy change of combustion,
ΔcH° trend
When comparing the ΔHc for successive members of a
homologous series such as alcohols there is a constant rise in
the value as the number of carbon atoms increases. The experimental results are lower than the calculated ones because there will be heat loss and incomplete combustion which leads to less energy being released.
why stir solution
so solid dissolves
to create a unifrom temp
what do we assume when doing enthalpy change
solution has a density of 1 g cm–3 (1)
mass of ammonium chloride / solid is ignored (1)
(specific) heat capacity of the solution is the same as water
why enthalpy chnage of combustion inaccurate
incomplete combustion evaporation of the ethanol/alcohol/fuel
give two reasons for the differenc btw book molar enthalpy change of combustion and bond enthalpy
mean bond enthalpy are averages/ mean for different molecules
butan-1-ol is a liquid and bond enthalpies refer to gases or mean enthalpy calcualtions don’t include changes of state
what would happen if larger Q used
if larger SHC taken Q will be larger so lowers the error in the final value obtained
why increase in enthalpy change of combustion from butane to pentane very small
butane is a gas and pentane is a liquid at 298K
liquids vapourise before combustion
pentane is a liquid so some of the enregy released by combustion is used to vaporise or more energy is needed to break intermolecular forces in pentane
why temp of solution measured for 3 min before adding the zinc
to ensure equilibration with the surroundings or
to take account of changing initial temperature of solution or to check that the emp is cosntant/stready
why temp measured over a period after zinc added
to allow for heat loss and cooling. temp is still changing
to allow the reaction to go to competion
• because it takes time to add 50 cm3 of sodium hydroxide
can SHC of water be used in reaction
Either • the specific heat capacity of acid/alkali is different from water/ there is salt/sodium sulfate (dissolved) so the specific heat capacity is different
• so the assumption is not valid Or
• ‘most’ of the acid and alkali solutions are water
• so the assumption is valid