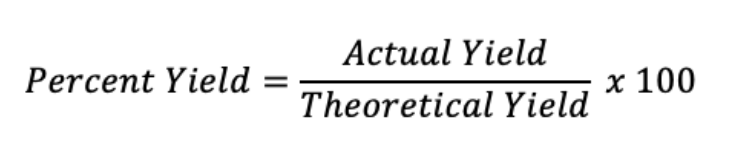PHYS SCI WW2 (Reaction Rates, Stoichiometry, and Energy Transformations)
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What is Reaction Rate?
The speed at which reactants turn into products.
Collision Theory: How do reactions happen?
According to Collision Theory, for a chemical reaction to occur, molecules must:
Collide with one another.
Have enough energy to break and form new bonds (called activation energy).
Be correctly oriented when they collide.
Imagine two people trying to high-five in a crowded room. If they don’t aim right (correct orientation) or hit too softly (not enough energy), the high-five won’t happen—just like a failed chemical reaction!
Factors that Affect Reaction Rate
Concentration (amount of reactant), Temperature, Particle Size/Surface Area, Catalyst
How does Concentration (Amount of Reactant) affect Reaction Rate?
More reactant particles = More collisions = Faster reaction
e.g. Higher acid concentration dissolves metals faster
How does Temperature affect Reaction Rate?
Higher temperature = More energy = More successful collisions
e.g. Cooking food at high heat speeds up the reaction
How does Particle Size/Surface Area affect Reaction Rate?
Smaller particles = More surface area for collisions = Faster reaction
e.g. Powdered sugar dissolves faster than a sugar cube
How does Catalyst affect Reaction Rate?
Lowers activation energy = Reaction happens faster
e.g. Enzymes in the body speed up digestion
What is a Catalyst?
A substance that speeds up a chemical reaction without being consumed in the process. It provides an alternative reaction pathway.
Types of Catalysts
Biological Catalysts (Enzymes) and Industrial Catalysts
Biological Catalysts (Enzymes)
Catalysts in living organisms
e.g. Amylase (breaks down starch in saliva) and Lactase (helps people digest lactose in dairy)
Industrial Catalysts
Used in manufacturing.
e.g. Platinum in car exhaust systems (converts toxic gases into harmless ones) and Fertilizers (helps plants grow faster)
Why do we need Chemical Calculations?
Chemical reactions are not random; they follow a fixed ratio based on the number of atoms involved. Using the wrong amounts of reactants leads to unwanted products, waste, or incomplete reactions.
Real-Life Examples of Chemical Calculations
In Laboratories: Scientists must measure precise amounts of reactants to create the correct compound with high purity.
In Industry: Companies calculate the exact amounts of reactants to maximize production efficiency and reduce costs.
In Medicine: Pharmaceuticals require precise chemical calculations to ensure the correct dosage of drugs is given to patients.
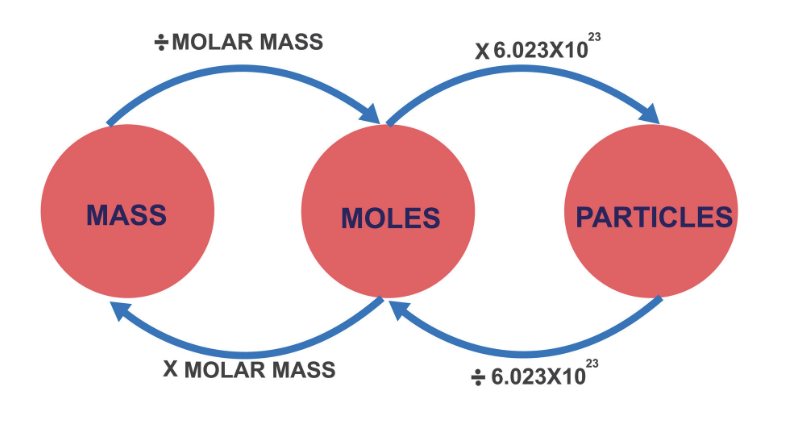
Avogadro’s number
1 mole of any substance contains 6.022 × 10²³ particles.
Mass ↔ Moles ↔ Particles Conversions
Stoichiometry
The study of the exact ratios between reactants and products in a chemical reaction
Why is Stoichiometry important?
Allows chemists to determine how much product can be made from a given amount of reactants.
Helps avoid excess waste in reactions.
How to do Stoichiometry?
Write the balanced equation
Convert Substance A to Substance B
Use the molar ratio from the balanced equation
Convert Substance B into the desired unit
Limiting Reactant
The substance that determines how much product can be formed in a chemical reaction.
Once it runs out, the reaction stops, and no more product is made.
Excess Reactant
The substance that is left over after the limiting reactant is used up
How to Identify the Limiting Reactant in a Chemical Reaction?
Convert the amount of each reactant to moles: Use the molar mass of each substance to convert grams to moles.
Use mole ratios to determine which reactant produces the least product: Compare how much product each reactant could form based on the balanced equation.
The reactant that forms the least amount of product is the limiting reactant.
How to Find the Mass of the Excess Reactant Left Over?
After identifying the limiting reactant, minus the amount that was actually needed by the amount that was given. Then, convert it to the desired unit.
Theoretical Yield
Maximum amount of product that can be produced from a given amount of reactants.
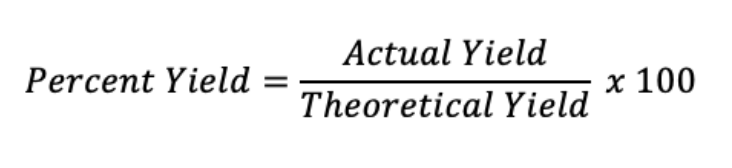
Percent Yield
Measures how much product is actually produced compared to the theoretical yield.