Biomarkers signature
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
Biomarker Signatures
•Many diseases (and the responses to pharmacotherapy) are characterised by
•Multiple genetic mutations
•Changes in gene expression
•Each of these changes is a potential biomarker.
The benefit of using data from multiple genes is that although an individual biomarker may be significantly different between the two groups (eg with and without the disease) it may not have very high predictive power when multiple biomarkers are combined in a signature the predictive power increases
Development of a BIOMARKER signature
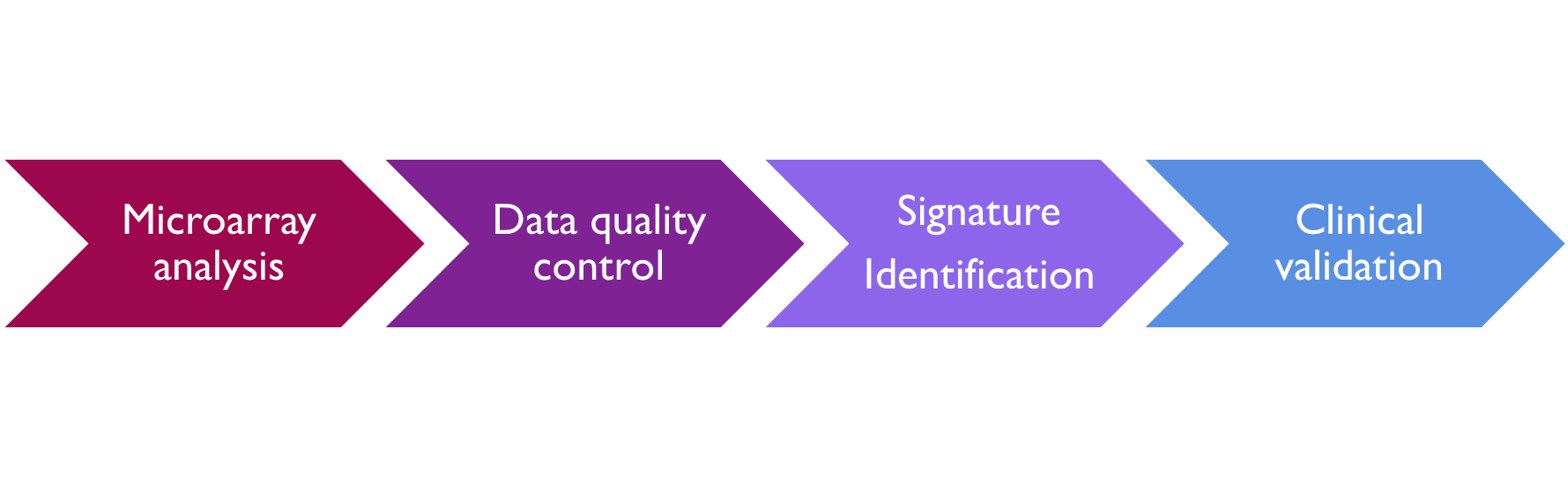
Microarray analysis - analysis of mRNA to look at the expression levels of genes in the control and experimental groups e.g. looking at genes that are over or under expressed in the experimental group compared to the control group, can also be used to look at different gene variations between the two groups. In both cases the result is a fluorescent image which is quantified.
the quality control aspect of this includes the determination of the threshold, of the signal and adjustment of the signal to noise ratio. And there are controls included on the microarray to enable this.
statistical analysis of the data set to identify markers that are different between the two groups, i.e. to identify genes are overexpressed or under expressed. Looking for a difference in expression
And then finally, there's a correlation analysis to identify associations of the signature with the phenotype and the phenotype could be as a disease subtype, It could be the drug response or it could be prognosis.
This now gives a biomarker signature, which must then undergo clinical validation. This is essentially a repeat of the analysis. But in a different patient population, a validation dataset is used to determine that relationship, observed in the analysis of the training data is maintained.
Oncotype DX Breast Cancer TEST
•A test to guide cytotoxic chemotherapy decisions in early breast cancer management for patients with early hormone positive HER-2 negative lymph node negative breast cancer
These patients will have endocrine hormone therapy and a decision needs to be made to see whether they also need cytotoxic chemotherapy.
•Aims to predict 10 year risk of distant breast cancer recurrence and chemotherapy benefit
•Value:
1.Avoidance of chemotherapy in those clearly identified as low risk (avoiding chemotherapy associated ADRs) -
2.Identification of those at intermediate and high risk of recurrence – informs therapy choice.
3.Confirms recurrence risk determined by clinical and pathological features of the tumour
Oncotype DX
Signature Development
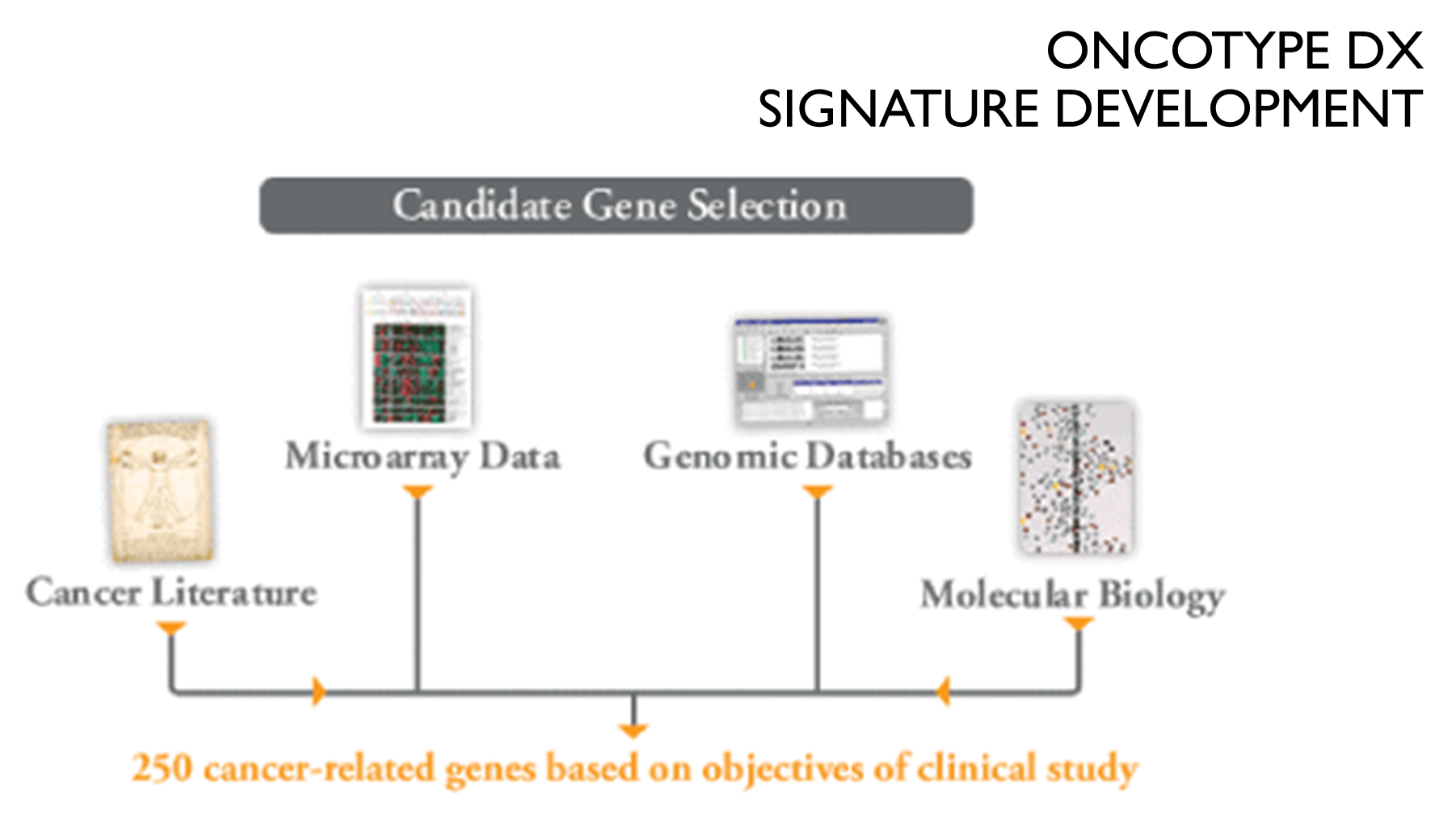
Oncotype DX
Gene Panel
These 250 candidate genes were analysed in a total of 447 patients in 3 independent clinical studies in order to identify a panel of genes that strongly correlated with distance recurrence free survival
•Biomarker signature of 21 genes
•16 breast cancer related - demonstrated a strong link between breast cancer recurrence.
•5 reference genes to normalise expression of these cancer related genes.
In addition, these studies were the basis for the recurrent score result calculation, which combines the gene expression data from this gene panel into a single result.
•Assay format: Real time quantitative RT-PCR
•Analysis of expression levels of the mRNA of the16 genes was normalised against the reference genes to give a total recurrence score - the change in each gene’s expression was scored from 0 to 15 where 1 unit increase was the doubling of the amount of RNA.
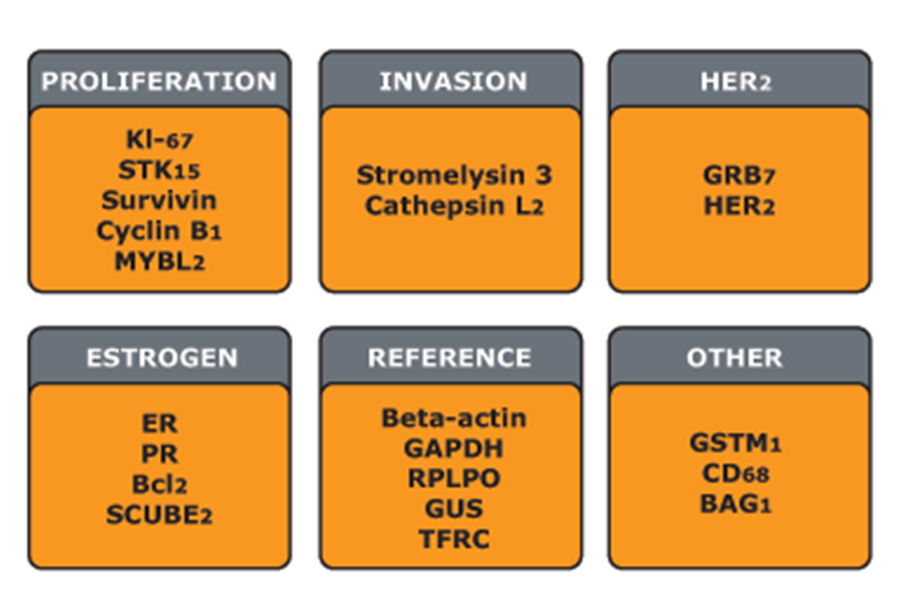
Each of the 16 genes was compared to the reference genes and the change in expression expressed as a value from nought. Fifteen were one unit increase was a doubling of the amount of RNA and this was used to calculate a reoccurrence score.
INITIAL Evaluation
of the oncotype DX assay
•Reoccurrence score: put into 3 categories
•low (<18)
•intermediate (>18 < 31)
•high (> 31)
•Look at distant recurrence at 10 years across patient groups stratified by age.
•For each age group a high reoccurrence score score is associated with higher % of recurrence
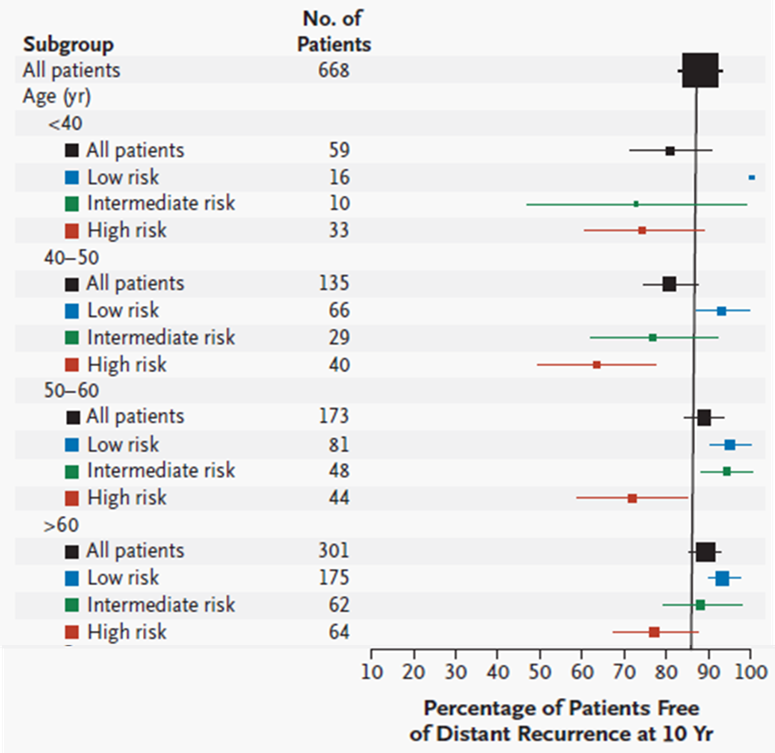
*The size of each square corresponds to the size of the subgroup; the horizontal lines represent the 95 percent confidence interval.
Further Evaluation of the oncotype DX assay
•Trial Assigning Individualized Options for Treatment (TAILORx) - prospective clinical trial to evaluate the benefit of chemotherapy in those with a midrange risk score (11-25)
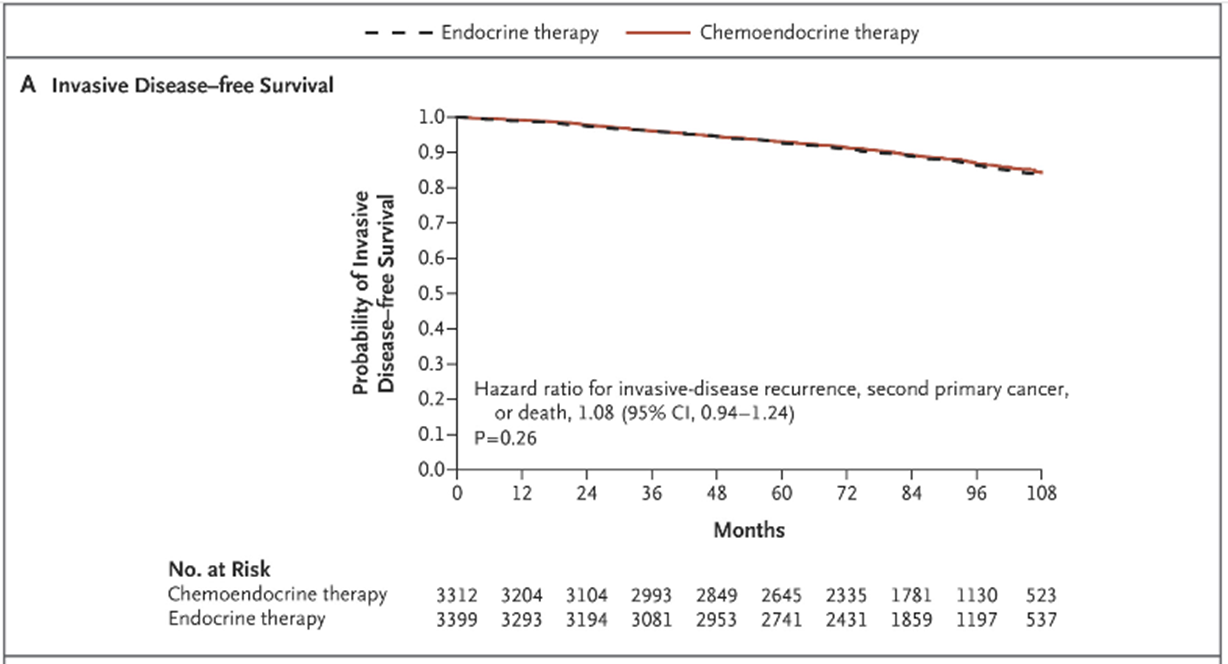
And they found that for this particular group of patients, there was no additional benefit of adding chemotherapy to endocrine and hormone therapy. There is no difference in the invasive disease free survival in either group. In other words, it didn't make any difference whether they had endocrine therapy on its own or endocrine therapy with chemotherapy.
Use of oncotype DX in practice
•NICE guidance on Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer (DG34) Dec 2018
•
•“Oncotype DX is recommended as an option for guiding adjuvant chemotherapy decisions for people with oestrogen receptor (ER)-positive human epidermal growth factor receptor 2 (HER2)-negative and lymph node (LN)-negative early breast cancer only if they an intermediate risk of distant recurrence using a validated tool such as PREDICT or the Nottingham Prognostic Index”
PredictSure-IBD
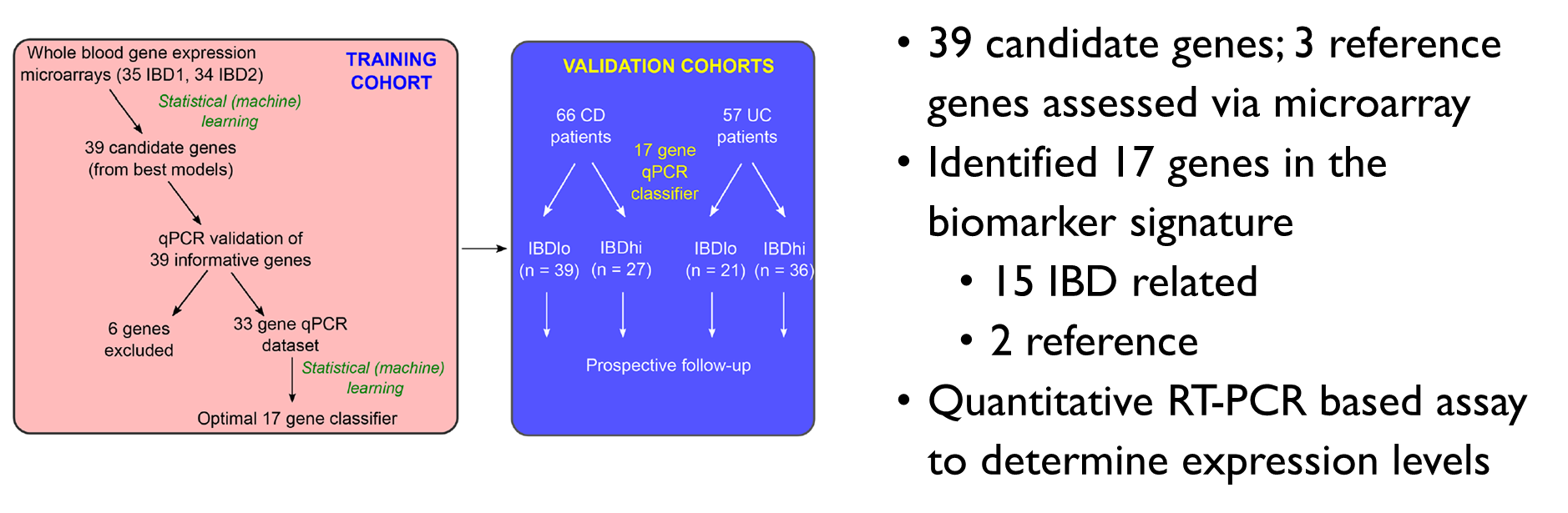
•39 candidate genes; 3 reference genes assessed via microarray
•Identified 17 genes in the biomarker signature
•15 IBD related
•2 reference
•Quantitative RT-PCR based assay to determine expression levels
Evaluation of the PREDICTSURE-IBD Assay
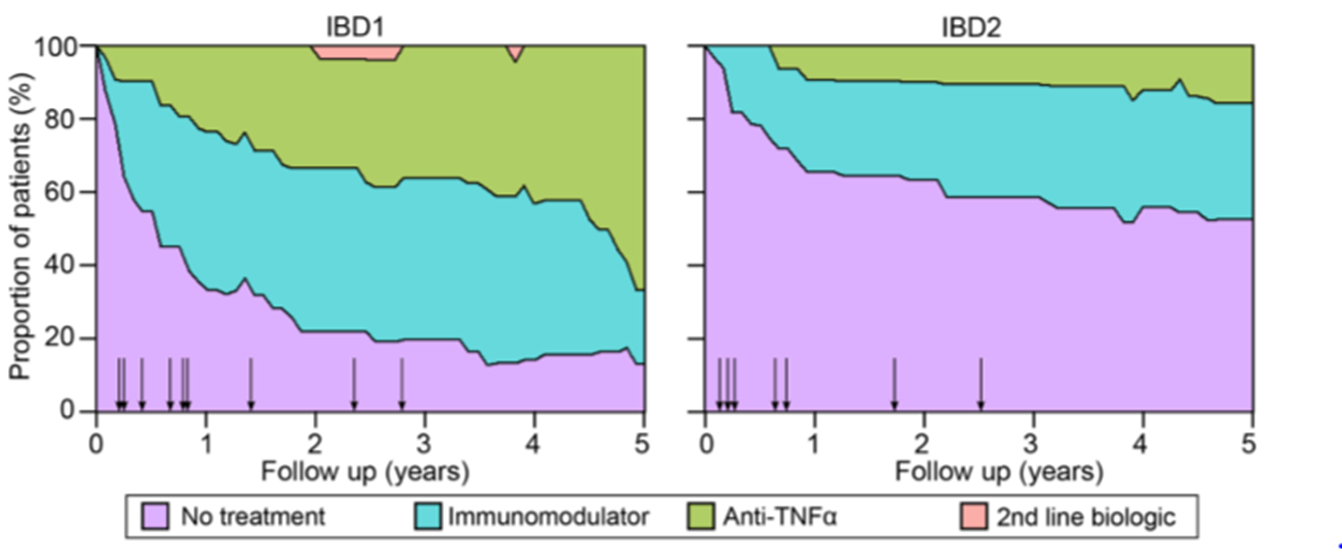
•The clinical course of Crohn’s disease is different in IBD1 and IBD2 Patients
•Stacked density plots demonstrating the maximum medical therapy that was required during 5 years’ prospective follow-up in the IBD1 and IBD2 subgroups. Treatments were plotted hierarchically (no treatment<immunomodulator <antiTNFα <second-line biologicals (vedolizumab or ustekinumab)).
•The assay is currently under review by NICE for use as a prognostic biomarker to guide therapy
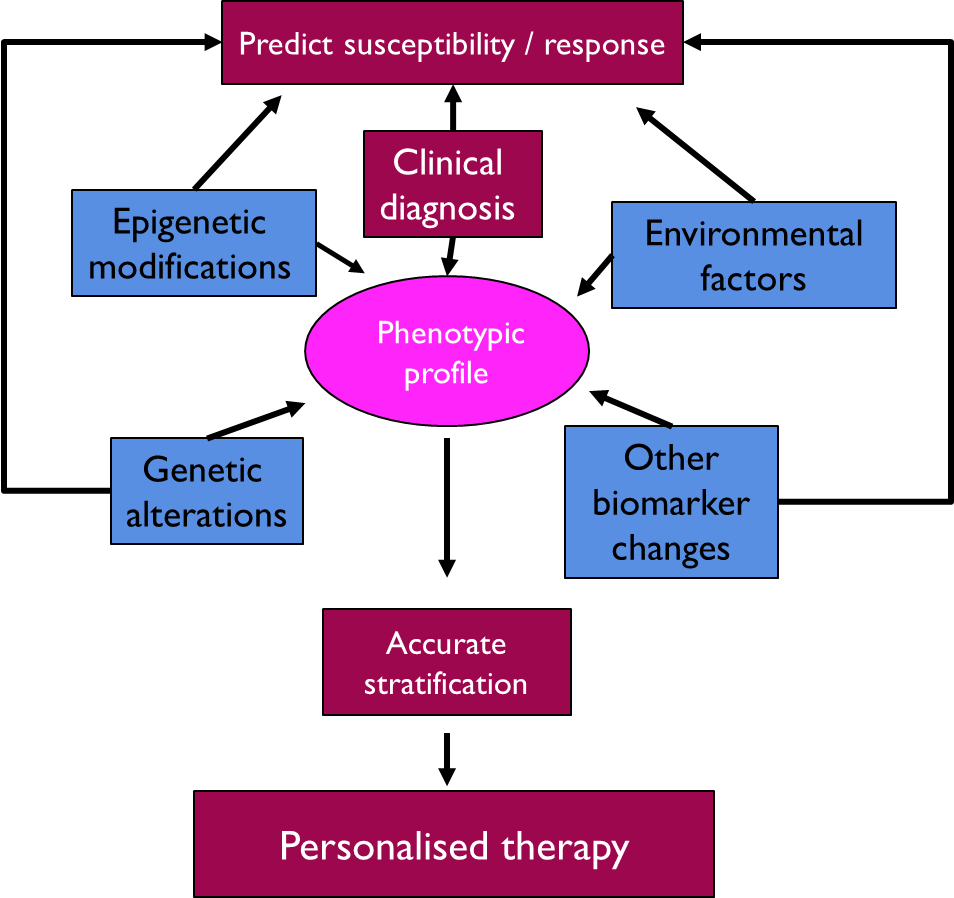
Role of Biomarkers
in clinical practice
•A variety of biomarkers contribute to a phenotypic profile and each contribution can vary between individuals giving rise to an individual phenotype
•Biomarkers can be used to stratify an individuals likelihood of an outcome and in term be used to personalise the therapy they receive