Quiz 3 (Chapters 4) Organic Chemistry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
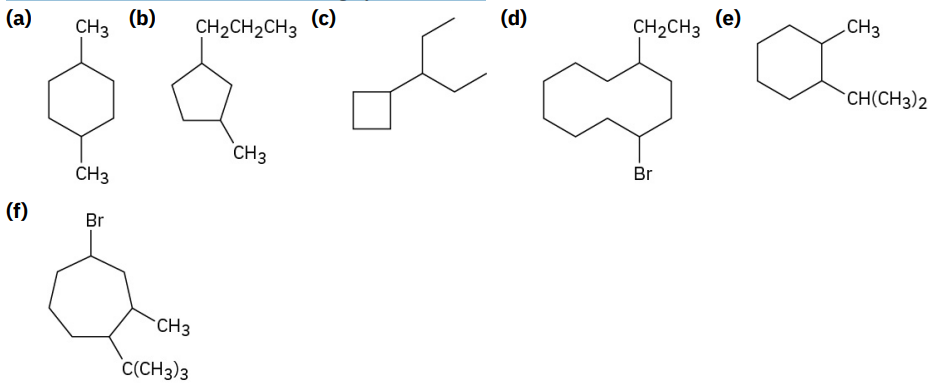
Give IUPAC names for the following cycloalkanes:
a) 1,4-dimethylcyclohexane
b) 1-methyl-3-propylcyclopentane
c) 3-cyclobutylpentane
d) 1-bromo-4-ethylcyclodecane
e) 1-isopropyl-2-methylcyclohexane
f) 4-bromo-1-tert-butyl-2-methylcycloheptane
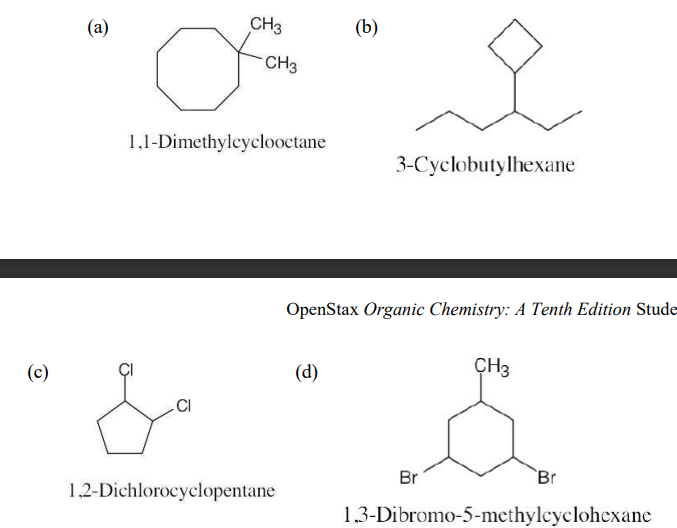
Draw structures corresponding to the following IUPAC names:
(a) 1,1-Dimethylcyclooctane
(b) 3-Cyclobutylhexane
(c) 1,2-Dichlorocyclopentane
(d) 1,3-Dibromo-5-methylcyclohexane
see image
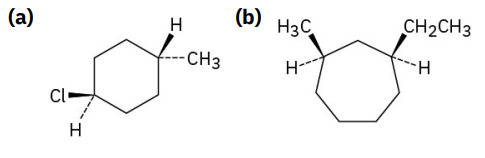
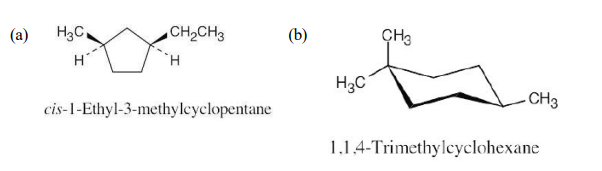
Name the following substances, including the cis- or trans- prefix:
see image
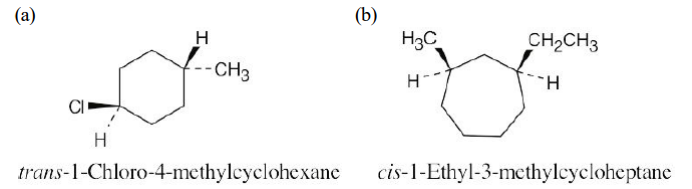
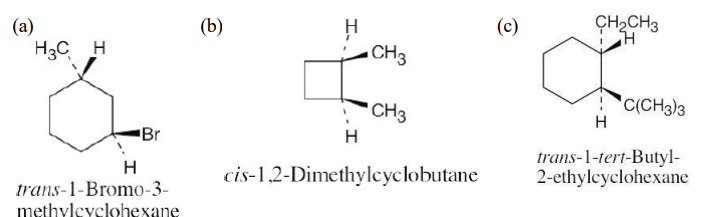
Draw the structures of the following molecules:
(a) trans-1-Bromo-3-methylcyclohexane
(b) cis-1,2-Dimethylcyclobutane
(c) trans-1-tert-Butyl-2-ethylcyclohexane
see image
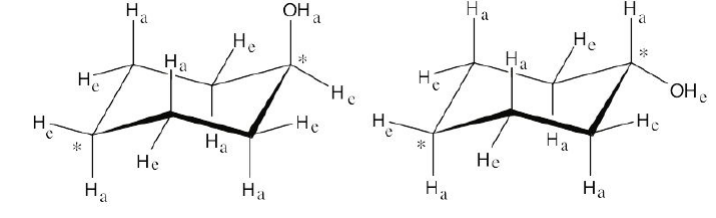
Draw two different chair conformations of cyclohexanol (hydroxycyclohexane), showing all hydrogen atoms. Identify each position as axial or equatorial.
see image
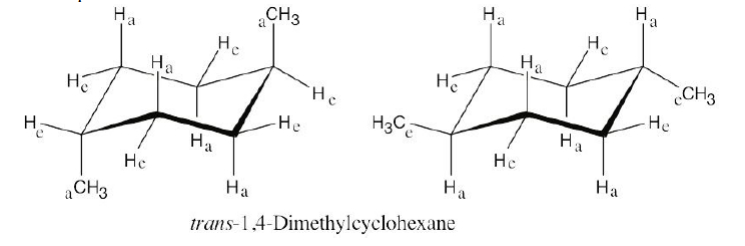
Draw two different chair conformations of trans-1,4-dimethylcyclohexane, and label all positions as axial or equatorial.
see image
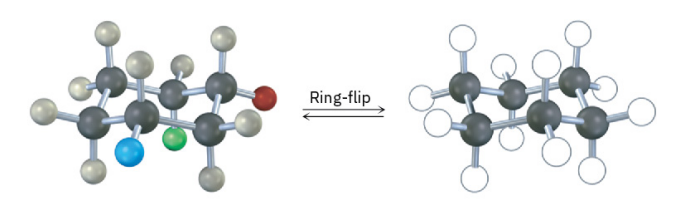
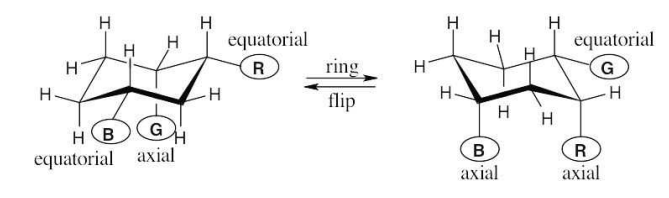
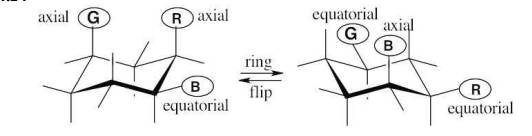
Identify each of the colored positions—red, blue, and green—as axial or equatorial. Then carry out a ring-flip, and show the new positions occupied by each color.
see image
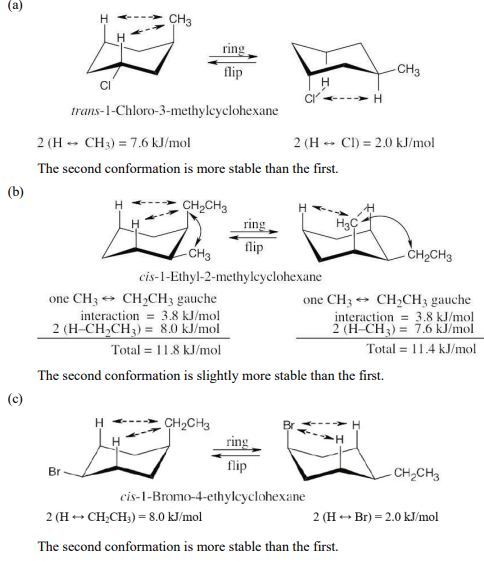
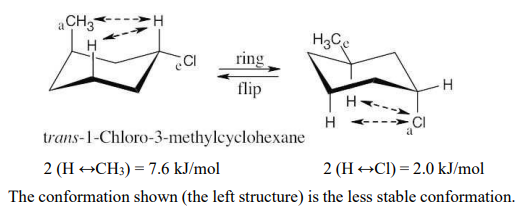
Draw the more stable chair conformation of the following molecules, and estimate the amount of strain in each:
(a) trans-1-Chloro-3-methylcyclohexane
(b) cis-1-Ethyl-2-methylcyclohexane
(c) cis-1-Bromo-4-ethylcyclohexane
(d) cis-1-tert-Butyl-4-ethylcyclohexane
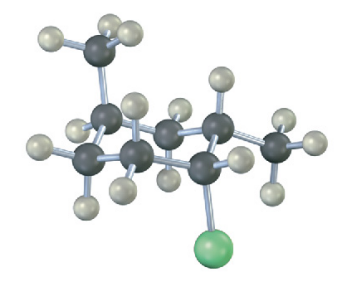
Identify each substituent in the following compound as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):
see image
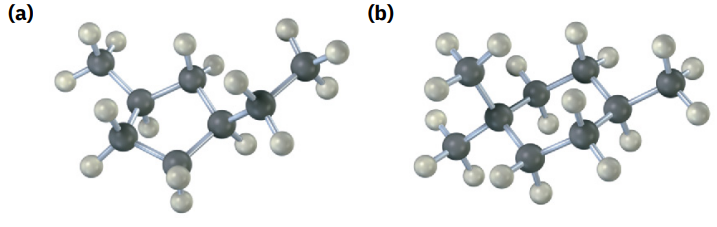
Name the following cycloalkanes:
see image
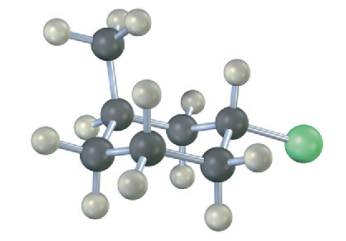
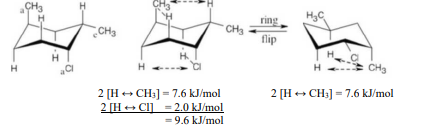
Name the following compound, identify each substituent as axial or equatorial, and tell whether the conformation shown is the more stable or less stable chair form (green = Cl):
see image
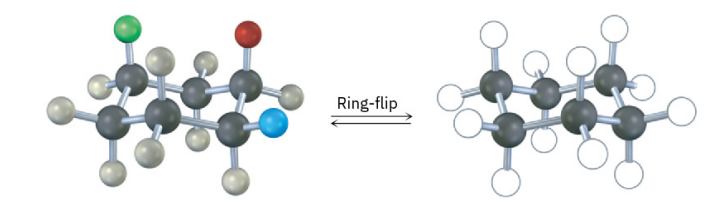
A trisubstituted cyclohexane with three substituents—red, green, and blue—undergoes a ring-flip to its alternate chair conformation. Identify each substituent as axial or equatorial, and show the positions occupied by the three substituents in the ring-flipped form.
see image
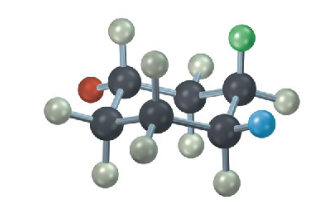
The following cyclohexane derivative has three substituents—red, green, and blue. Identify each substituent as axial or equatorial, and identify each pair of relationships (red–blue, red–green, and blue–green) as cis or trans.
see image

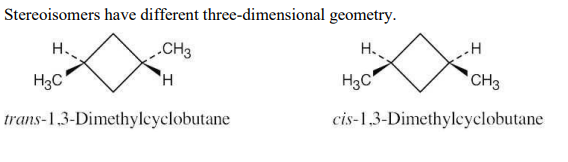
Draw a stereoisomer of trans-1,3-dimethylcyclobutane.
see image

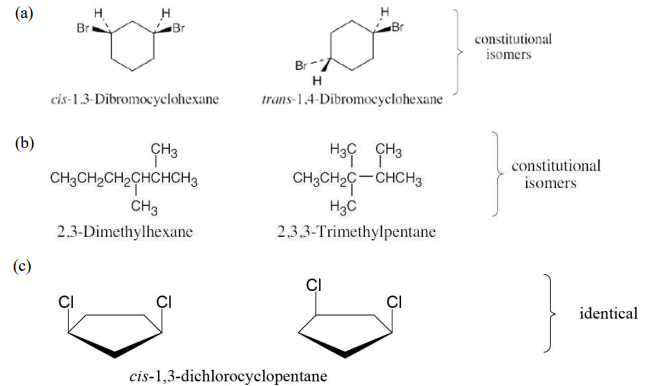
Tell whether the following pairs of compounds are identical, constitutional isomers, stereoisomers, or unrelated.
(a) cis-1,3-Dibromocyclohexane and trans-1,4-dibromocyclohexane
(b) 2,3-Dimethylhexane and 2,3,3-trimethylpentane
see image
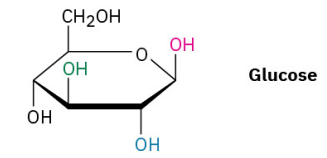
Identify each pair of relationships among the –OH groups in glucose (red–blue, red–green, red–black, blue–green, blue–black, green–black) as cis or trans.
see image
