Chemistry 1 - Lesson 1: Atomic Mass and Exponential Decay
Lesson 1: Atomic Mass
Lesson 1: Atomic Mass
NOTE: Quizlet cards marked with CRB indicates that these flashcards cover the content found in the Content Review Books. Cards not marked with CRB cover the video playlist content. Enjoy! :)
NOTE: Quizlet cards marked with CRB indicates that these flashcards cover the content found in the Content Review Books. Cards not marked with CRB cover the video playlist content. Enjoy! :)
1/36
Earn XP
Description and Tags
Chemistry 1 Lesson 1
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
Lesson 1: Atomic Mass
Lesson 1: Atomic Mass
NOTE: Quizlet cards marked with CRB indicates that these flashcards cover the content found in the Content Review Books. Cards not marked with CRB cover the video playlist content. Enjoy! :)
NOTE: Quizlet cards marked with CRB indicates that these flashcards cover the content found in the Content Review Books. Cards not marked with CRB cover the video playlist content. Enjoy! :)
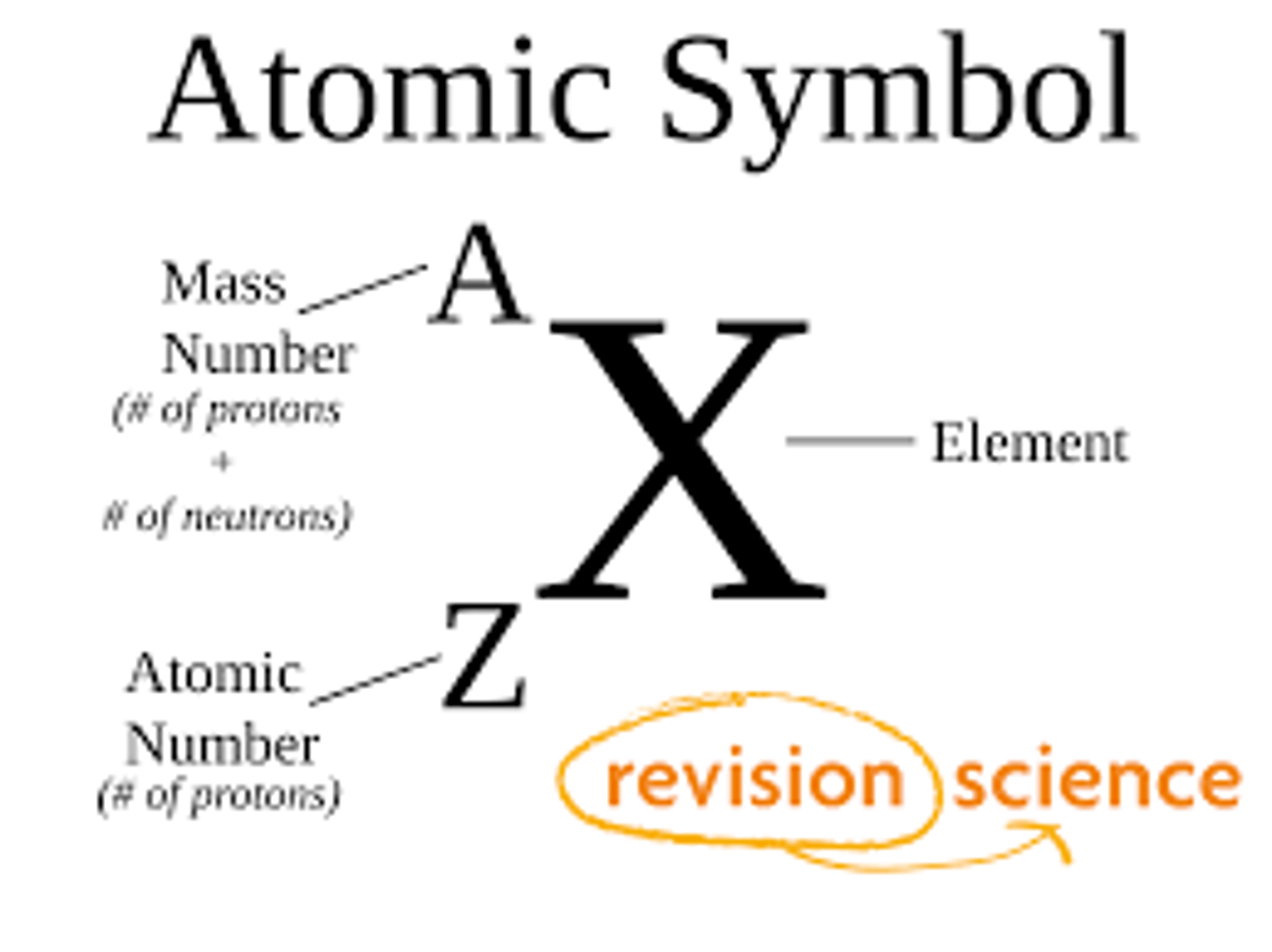
Correctly match the following terms with their abbreviation:
I. Mass number
II. Atomic number
III. Number of neutrons
(A) A
(B) M
(C) N
(D) Z
I. (A)
II. (D)
III. (C)
Mass number- A
Atomic number- Z
Number of neutrons- N
Hydrogen has an atomic number of 1 and an atomic mass of 1. Deuterium has an atomic number of 1 and an atomic mass of 2. How many protons does Hydrogen have compared with Deuterium?
Hydrogen has 1 proton as its atomic number is 1.
Deuterium also has 1 proton as its atomic number is also 1.
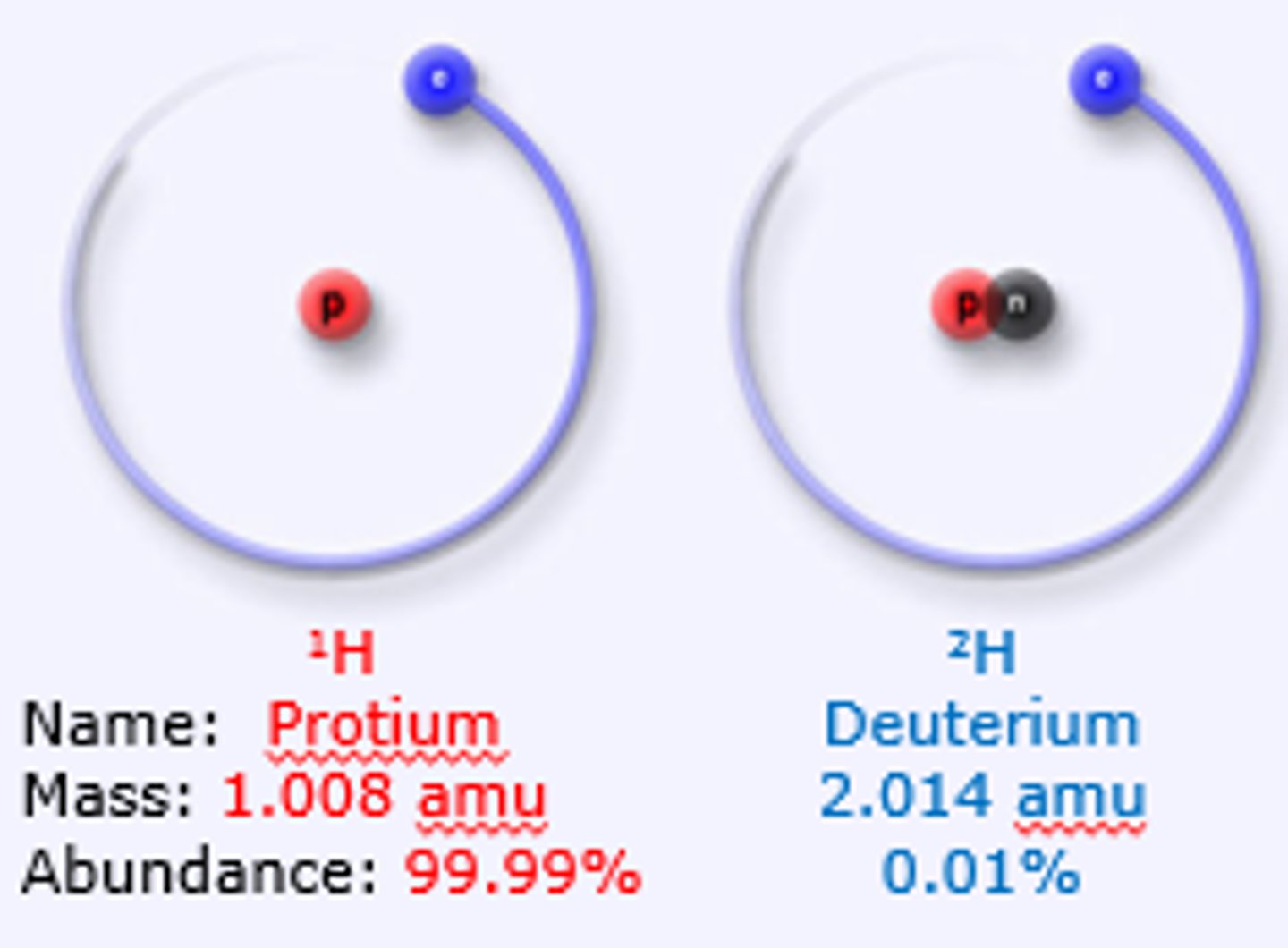
What is responsible for the extra mass within Deuterium as compared to Hydrogen?
Deuterium has an extra neutron compared to Hydrogen.
Deuterium and Hydrogen are related in that they are:
(A) Isomers
(B) Isotropes
(C) Isotonic compounds
(D) Isotopes
(D) Isotopes
Isotopes are similar in atomic number but different in terms of their atomic mass, due to differing numbers of neutrons.
CRB An atom with an atomic mass of 161 and 87 neutrons is an isotope of what element? (see periodic table here: https://www.ptable.com/Images/periodic%20table.png)
(A) Francium
(B) Dysporium
(C) Terbium
(D) Tungsten
(D) Tungsten
161 neutrons and protons - 87 neutrons = 74 protons
Consulting the periodic table, the element with 74 protons is Tungsten (W).
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
Uranium has an atomic number of 92 and an atomic mass of 235. How many neutrons does Uranium contain?
(A) 92
(B) 110
(C) 143
(D) 235
(C) 143
235 neutrons and protons - 92 protons = 143 neutrons
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB Compare atomic mass and atomic weight.
The atomic mass of an atom is a discrete number based on the number of nucleons in a single atom.
The atomic weight of an element is a weighted average of the atomic masses of all atoms of that element in nature.
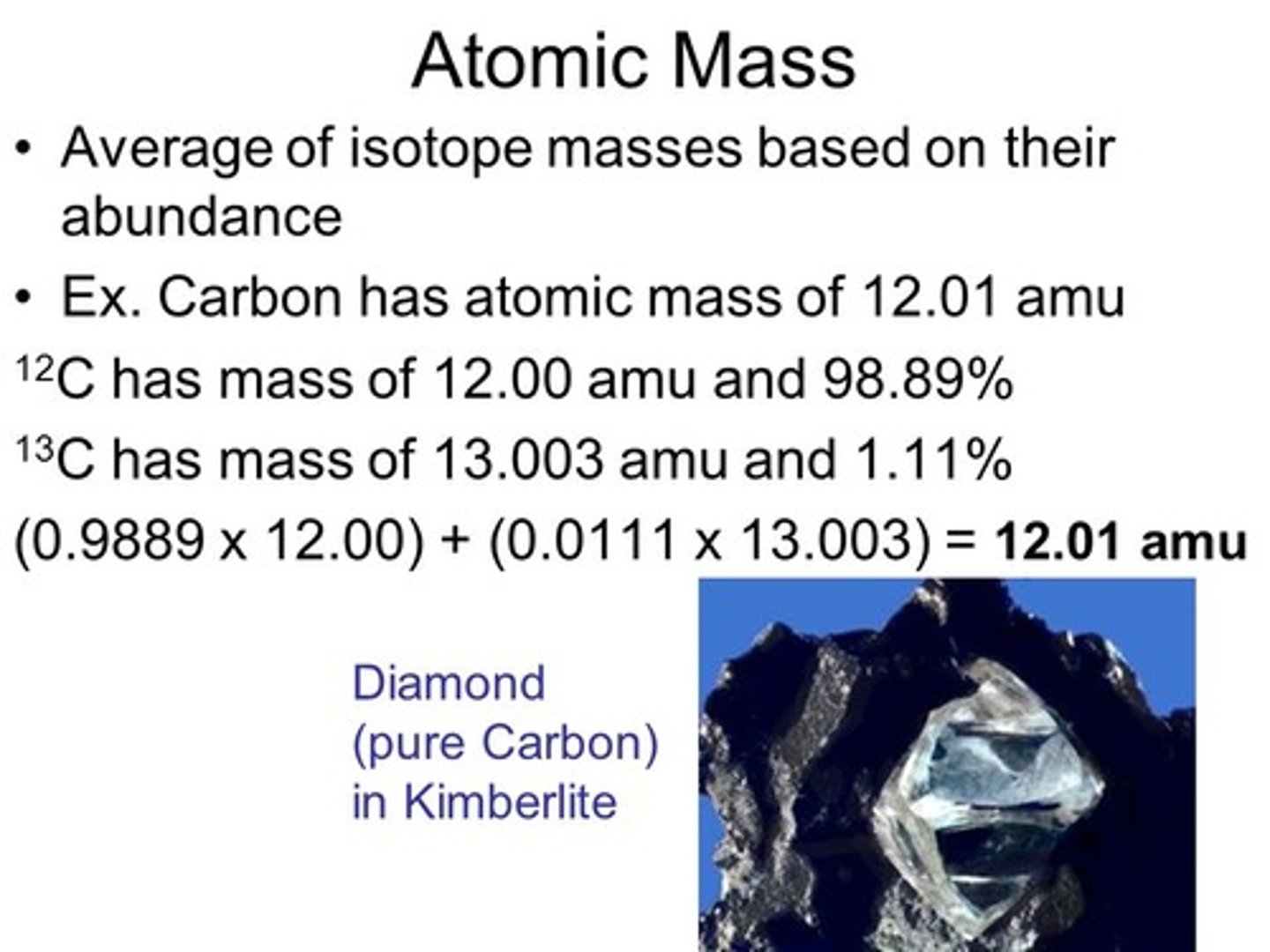
Why is the atomic weight of Carbon reported as 12.01 instead of 12?
Because not all Carbon in nature is the Carbon-12 isotope. You have to account for the Carbon-13 that exists out there. The atomic weight is a weighted average.
What percent of Carbon atoms out there are Carbon-12 if the atomic weight of Carbon is 12.01?
About 99 percent of Carbon in nature is Carbon 12.
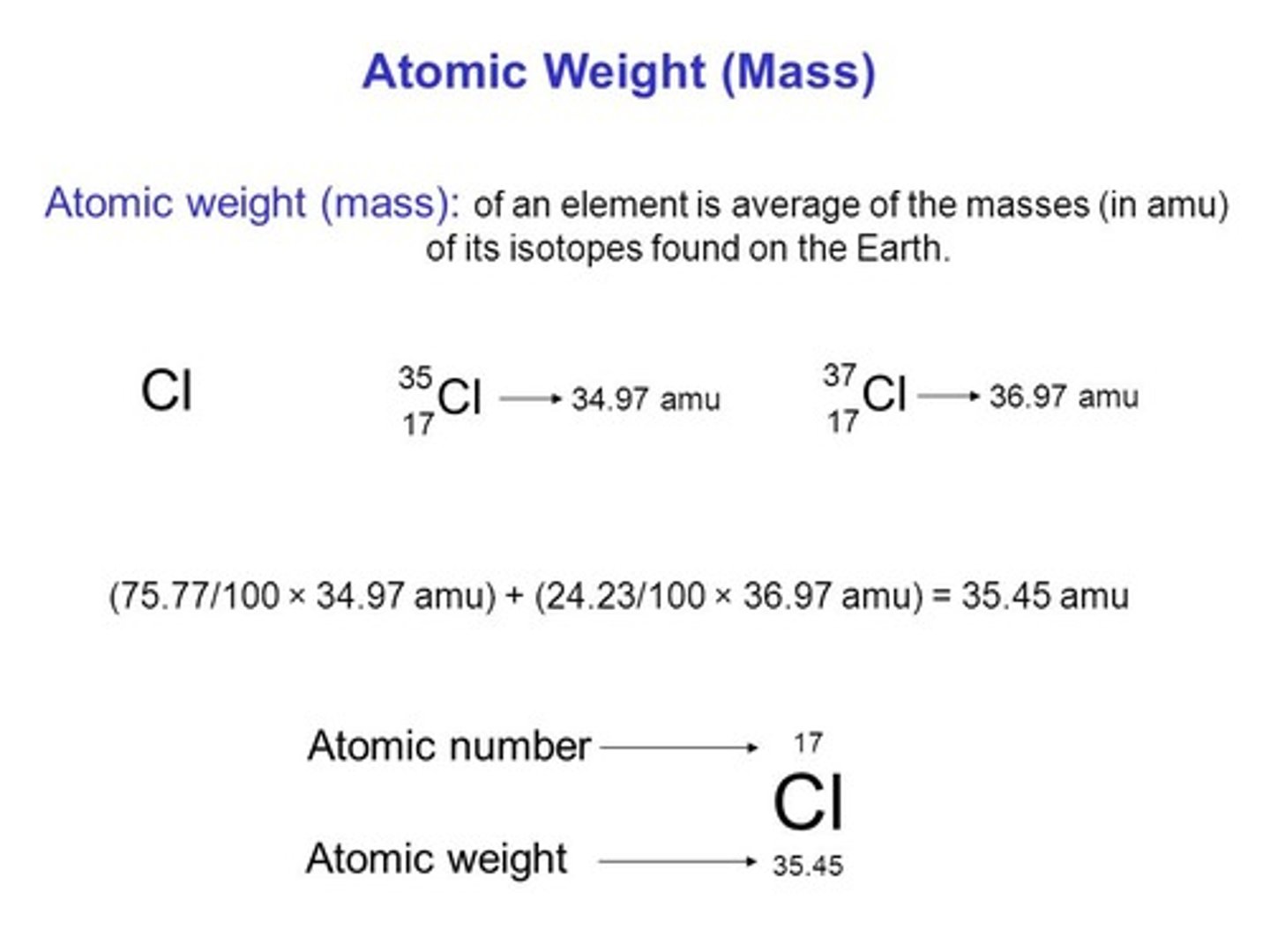
CRB What percentage of Chlorine atoms are Chlorine-35 (34.97 amu) and Chlorine-37 (36.97 amu) if these are the only two isotopes that exist and the atomic weight is 35.45 amu?
(A) 26.58% Chlorine-35; 74.42% Chlorine-37
(B) 50.35% Chlorine-35; 49.65% Chlorine-37
(C) 75.77% Chlorine-35; 24.23% Chlorine-37
(D) 90.21% Chlorine-35; 9.79% Chlorine-37
(C) 75.77% Chlorine-35; 24.23% Chlorine-37
34.97(x) + 36.97 (1-x) = 35.45
2x = 1.52
x = 0.76 = 76%
Approximately 75% of the chlorine is Chlorine-35 and 25% is Chlorine-37.
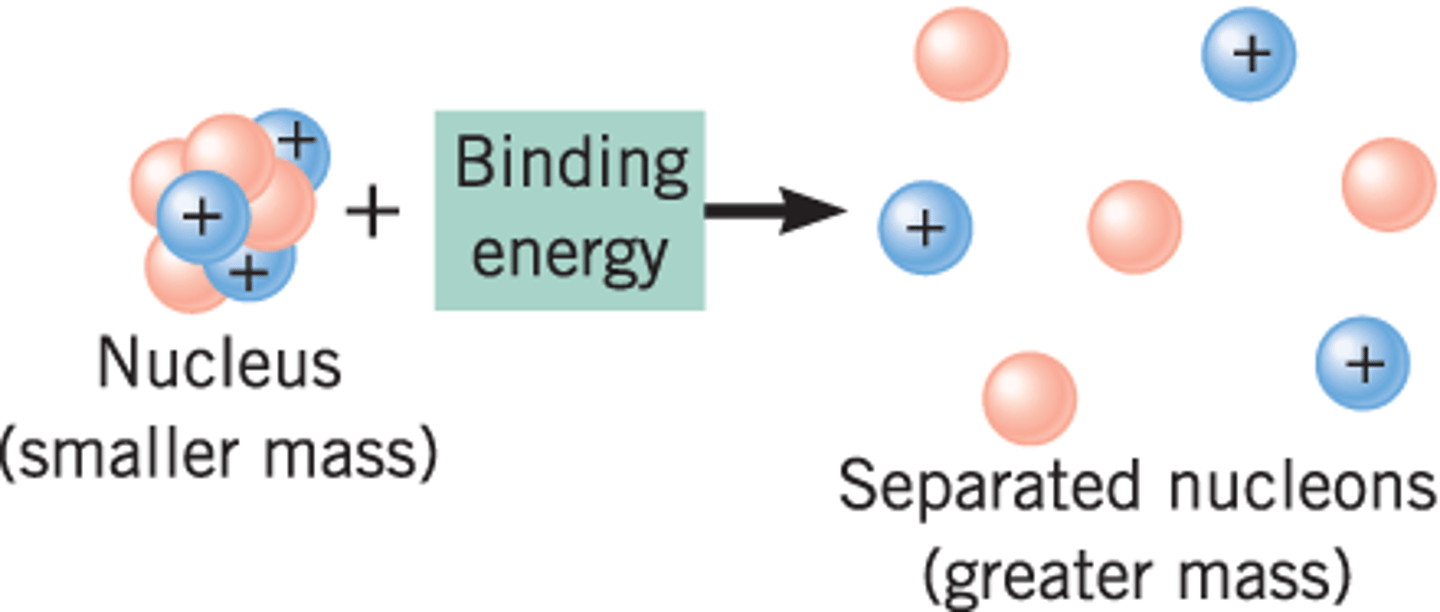
What accounts for the actual mass of Helium being .03 amu less than its predicted mass (the mass expected based on the individual masses of its protons, neutrons and electrons)?
The .03 amu is a result of the mass being converted into energy as the protons, neutrons, and electrons are brought together to form the atom.
The actual mass of Helium is .03 amu less than its predicted mass (the mass expected based on the individual masses of its protons, neutrons and electrons). In the case of Helium, .03 amu is considered the:
(A) Binding energy
(B) Atomic mass
(C) Atomic mass difference
(D) Mass defect
(D) Mass defect
The mass defect is the mass difference between the actual and predicted mass of an atom.
What equation for energy equates a change in mass to a change in energy?
E= mc^2
E = Energy
m = mass
c = speed of light = 3⋅10^8 m/s
If there is a 0.03 amu mass defect of Helium, there are 1.66054 ⋅ 10^-27 kg per amu, and the speed of light is 2.99792 ⋅ 10^8, what is the binding energy for Helium?
(A) 1.2 ⋅ 10^-6 J
(B) 5.8 ⋅ 10^-9 J
(C) 4.5 ⋅ 10^-12 J
(D) 9.4 ⋅ 10^-14 J
(C) 4.5 ⋅ 10^-12 J
(.03 amu )⋅(1.66054 ⋅ 10^-27 kg/amu) = 5 ⋅ 10^-29 kg
E = mc^2
E = (5 ⋅ 10^-29)((3 ⋅ 10^8)^2)
E = 4.5 ⋅ 10^-12 J
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/
CRB What is Avogadro's number equal to, and how does it relate the number of atoms of an element to moles?
Avogadro's number (NA)= 6.02 ⋅ 10^23 mol^-1
Avogadro's number is the number of atoms/molecules in a mole, so multiplying Avogadro's number by the moles of an element gives the number of atoms of that element.

CRB If the atomic weight of Xenon is 131.3, how much does 10^22 atoms of Xenon weigh in grams?
(A) 1.31 g
(B) 2.18 g
(C) 4.97 g
(D) 11.31 g
(B) 2.18 g
10^22/(6.02 ⋅ 10^23 mol^-1) = approx 1.5 ⋅ 10^-2 mol (actual 0.0166 mol Xenon)
0.0166mol ⋅ 131.3 g/mol = approximately 2.5g Xenon (actual 2.18g Xenon)
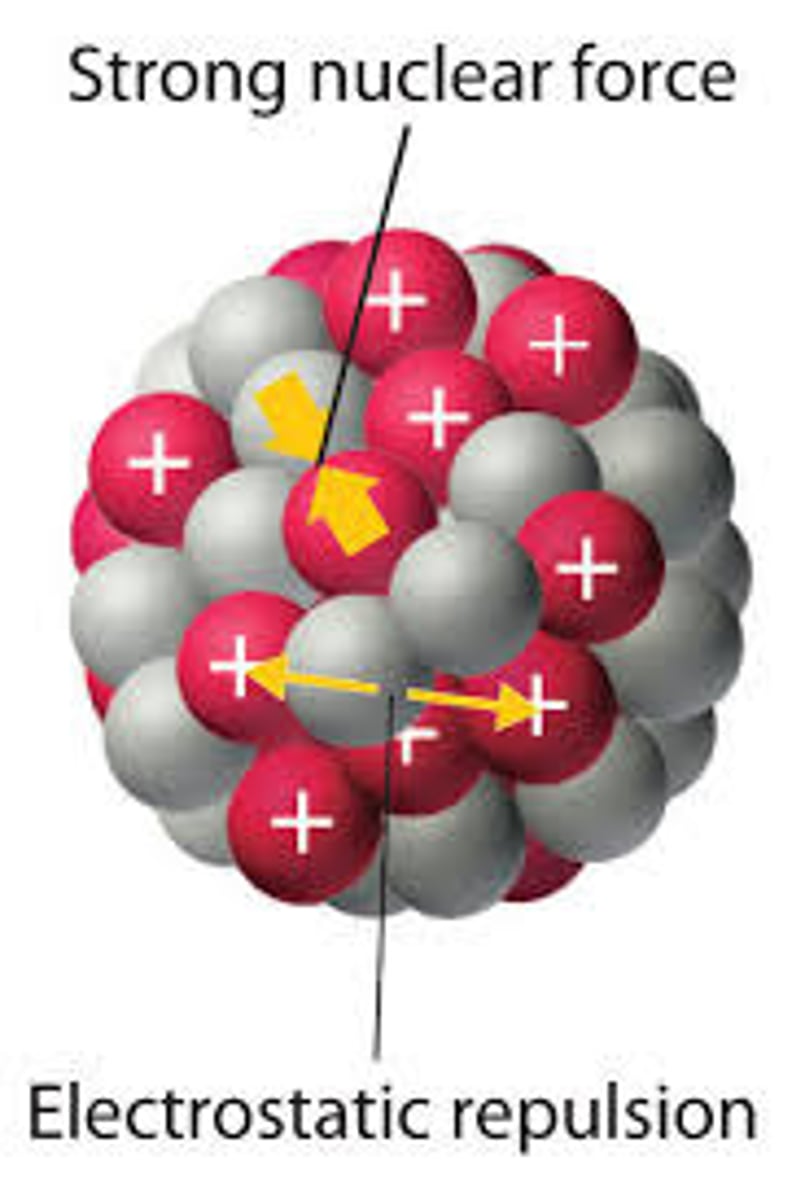
The nuclear strong force accounts for what phenomenon?
The nuclear strong force accounts for the fact that the protons in the nucleus are both positively charged and yet are not repelling each other.
Which of the following are considered nucleons?
I. Protons
II. Neutrons
III. Electrons
(A) I Only
(B) I and II Only
(C) II and III Only
(D) I, II, and III
(B) I and II Only
Protons and neutrons are both considered nucleons as they are both found in the nucleus of an atom.
CRB When describing the mass number of an atom, the units used are __________. One of these units can describe the mass of ____________.
(A) amu, protons
(B) amu, neutrons
(C) amu, nucleons
(D) grams, neutrons
(C) amu, nucleons
When describing the mass number of an atom, the units used are atomic mass units (amu). One of these units can describe the mass of nucleons (both protons and neutrons).
CRB True or false? Although not a nucleon, an electron's mass is also approximately 1 amu.
False. An electron's mass is about 1/2000th of an amu, making it significantly less massive than a nucleon.
Which is stronger? The electrostatic force between the protons in a nucleus, or the nuclear strong force?
The nuclear strong force, which is why the protons are not repelled away from each other.
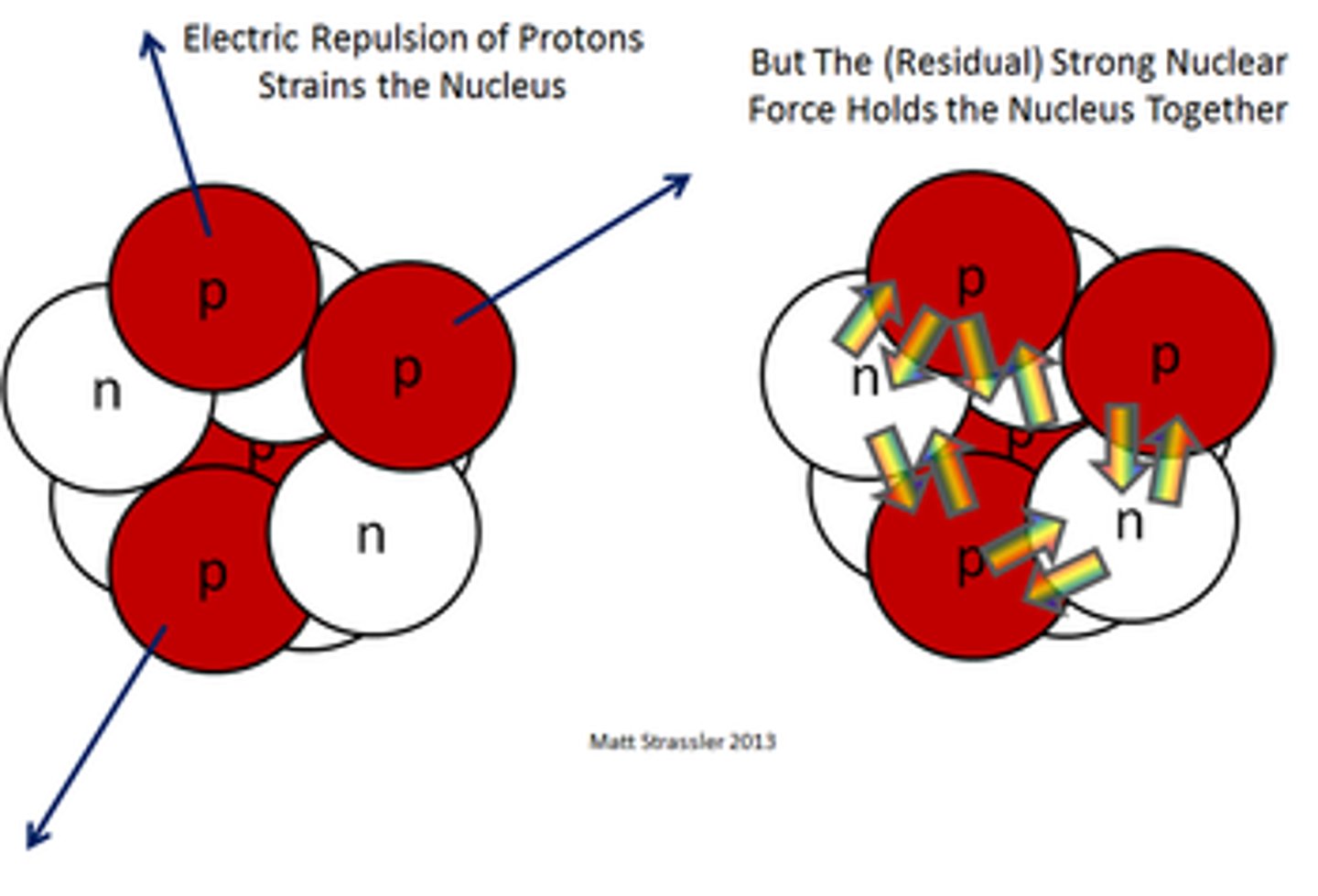
Which only acts over short distances? The electrostatic force between the protons in a nucleus, or the nuclear strong force?
The nuclear strong force only acts over short distances.
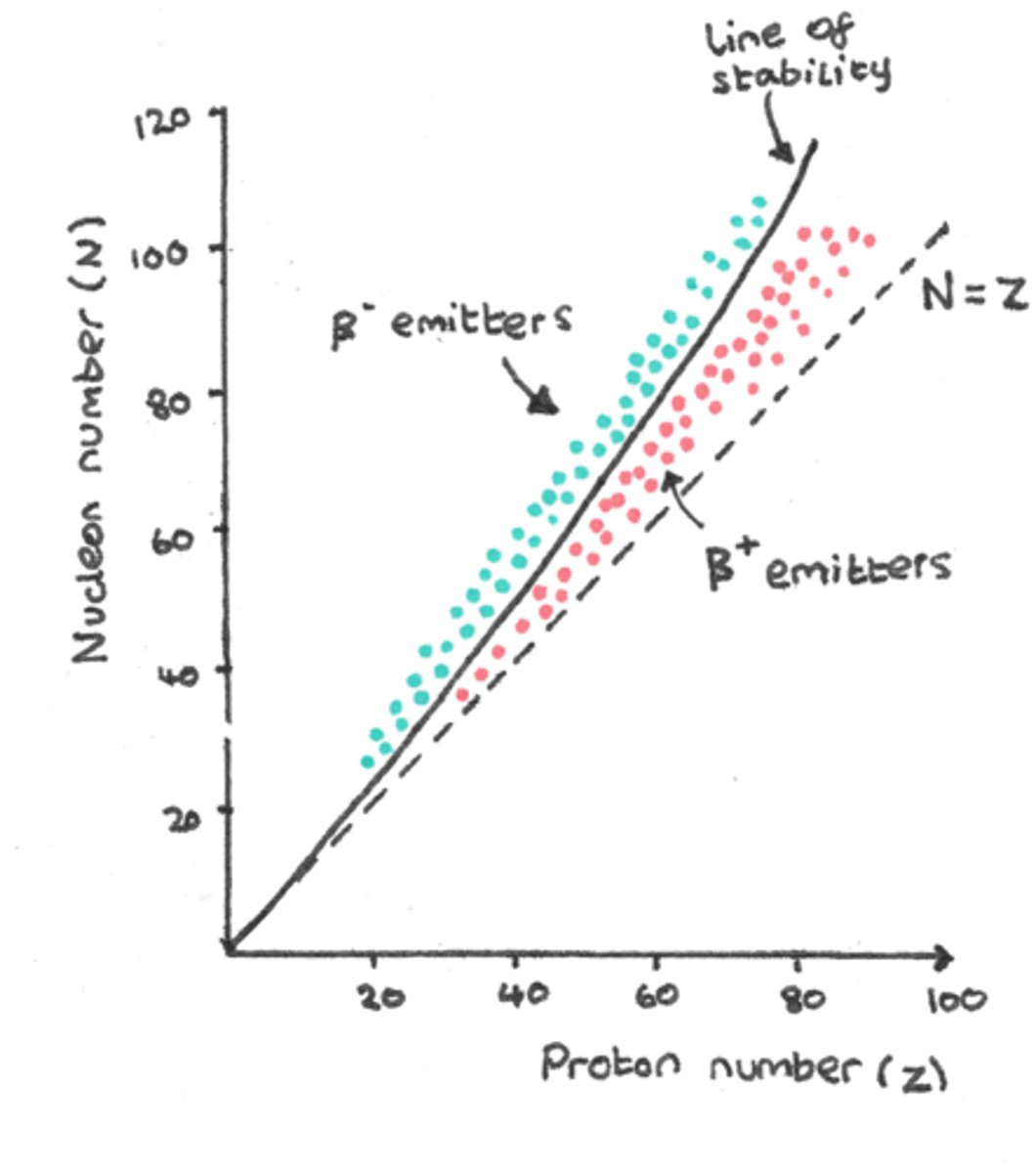
The N/Z ratio of Carbon-14 is 4/3. What does this indicate concerning the stability of Carbon-14?
It is unstable and will undergo radioactive decay.
The stable N/Z ratio for large atoms (Z > 83) is greater or less than that of small atoms? Why?
The stable N/Z ratio for large atoms is greater than that of small atoms. This is due to the fact that once you add more nucleons to a nucleus, the protons on opposite ends of the nucleus will not experience the strong nuclear force; thus, protons will be more repelled from one another, resulting in less protons per neutrons in the nucleus.
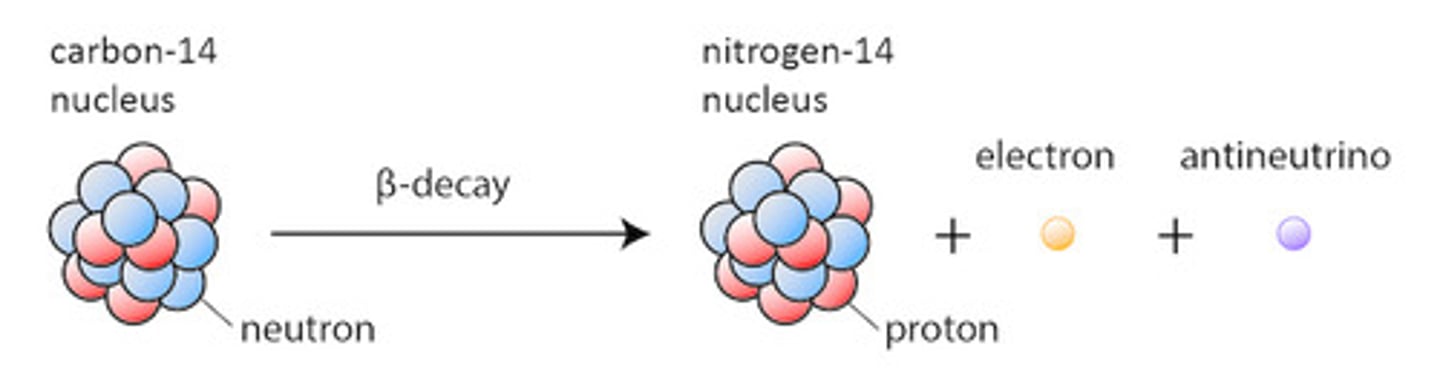
Carbon-14 undergoes radioactive decay, forming Nitrogen-14 and what other particle? This is an example of?
(A) Positron, positron emission
(B) Alpha particle, alpha-decay
(C) Electron, beta-decay
(D) Photon, gamma-decay
(C) Electron, beta-decay
Carbon-14 would emit an electron, resulting in the conversion of one neutron into a proton.
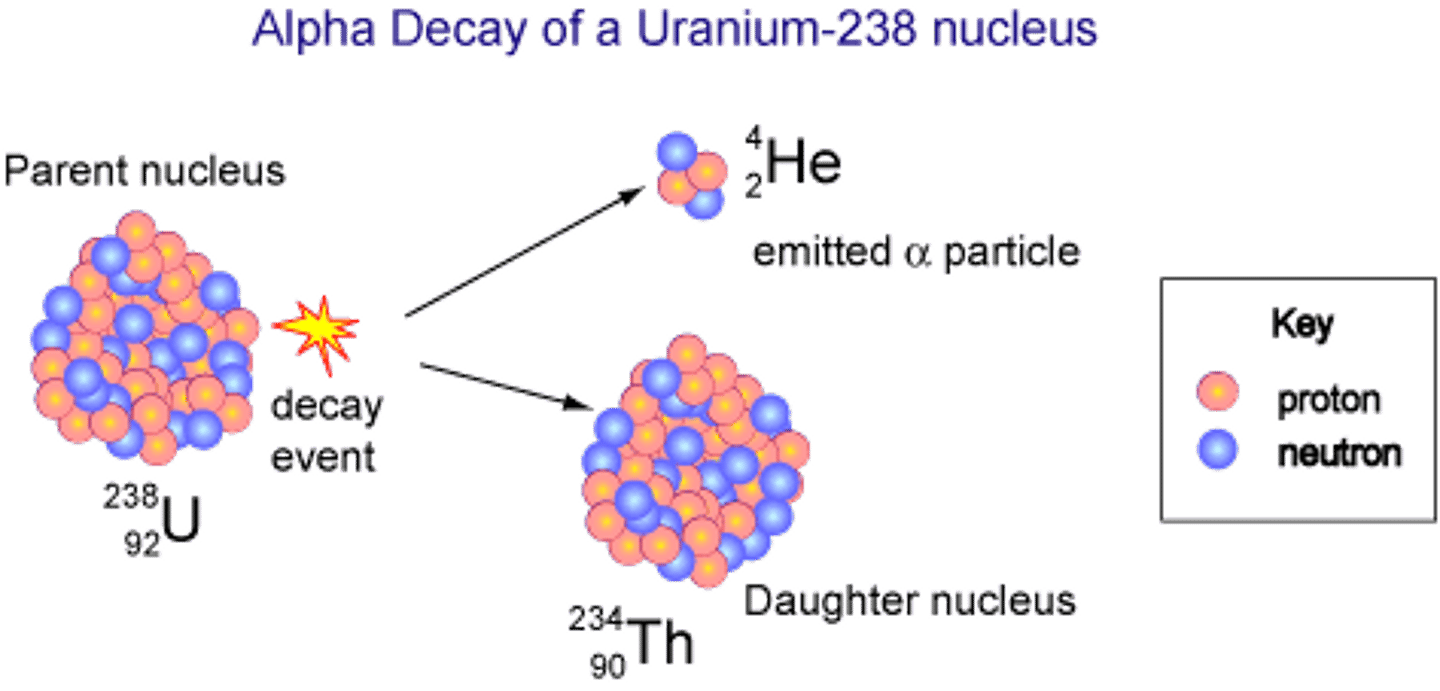
Uranium-238 undergoes radioactive decay, forming Thorium-234 and what other particle? This is an example of?
(A) Positron, positron emission
(B) Alpha particle, alpha-decay
(C) Electron, beta-decay
(D) Photon, gamma-decay
(B) Alpha particle, alpha-decay
Technitium-99 undergoes radioactive decay, forming Technitium-99 (with the same number of protons) and what other particle? This is an example of?
(A) Positron, positron-emission
(B) Alpha particle, alpha-decay
(C) Electron, beta-decay
(D) Photon, gamma-decay
(D) Photon, gamma-decay
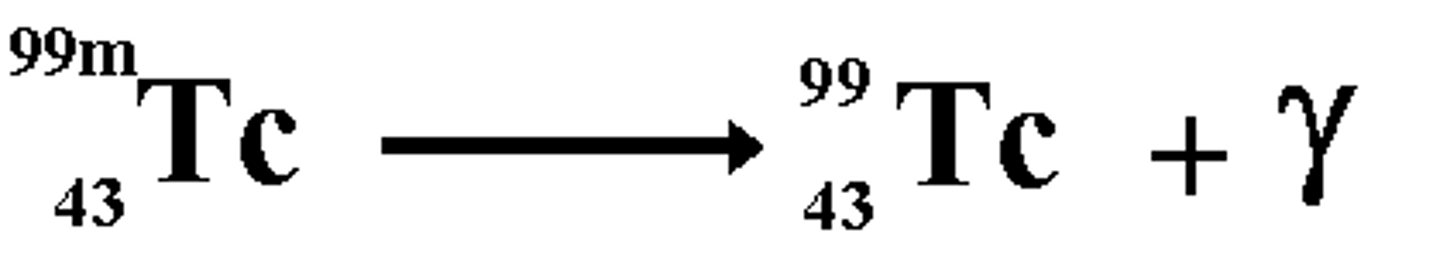
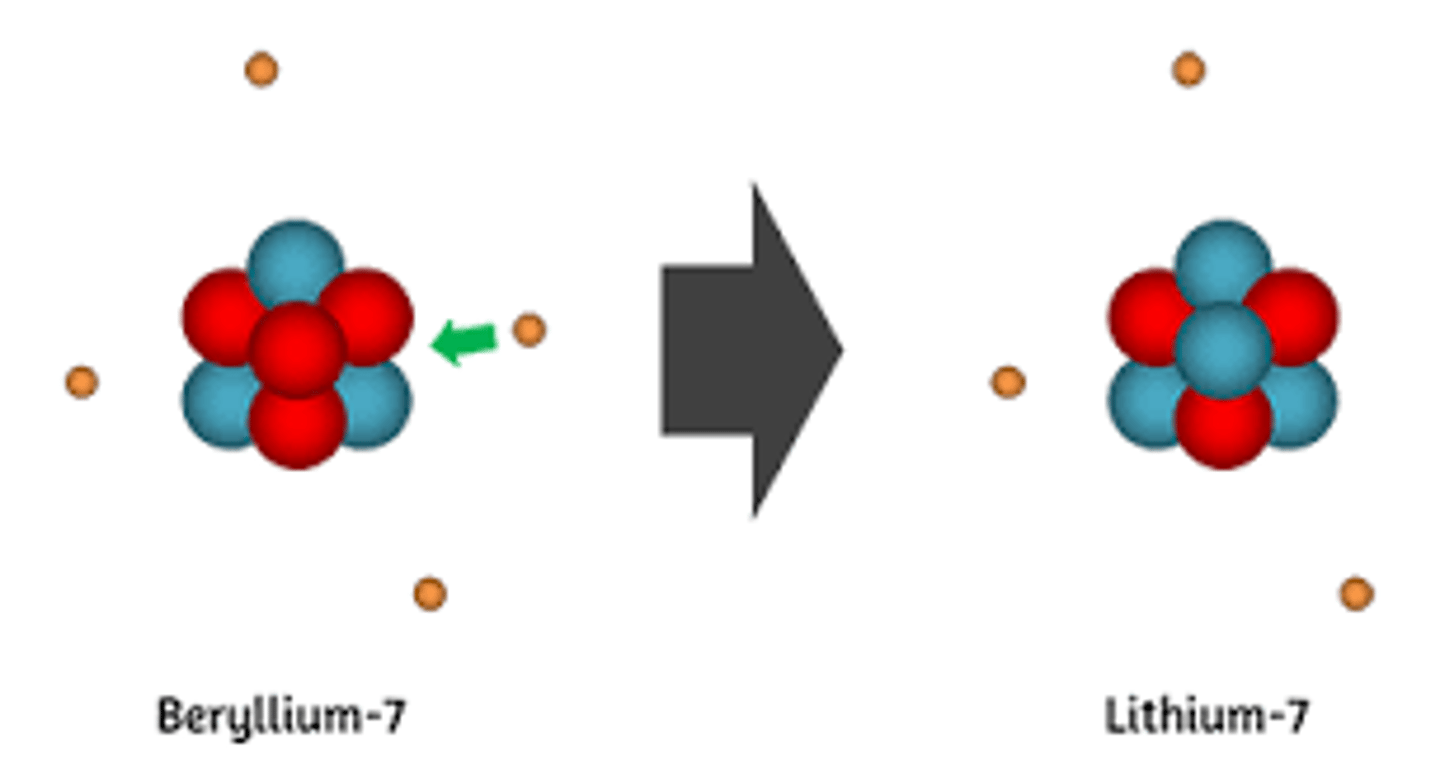
Berylium-7 undergoes radioactive decay, forming Lithium-7 (Berylium-7 with one less proton and one more neutron) and what other particle? This is an example of?
(A) Positron, positron-emission
(B) Alpha particle, alpha-decay
(C) Electron, beta-decay
(D) Photon, gamma-decay
(A) Positron, positron-emission
CRB Electron capture is another form of radioactive decay, where a proton is converted to a neutron. Electron capture could be considered the reverse process of which aforementioned radioactive decay type?
(A) Positron emission
(B) Alpha-decay
(C) Beta-decay
(D) Gamma-decay
(C) Beta-decay
In Beta-decay, a neutron is split into a proton and an electron that is emitted.
In Electron capture, an electron is taken into the nucleus to turn a proton into a neutron.
CRB A positively charged atom is called a _________, whereas a negatively charged atom is called a ____________.
(A) Positron, electron
(B) Cation, anion
(C) Anion, cation
(D) Electron, positron
(B) Cation, anion
A positively charged atom is called a cation, whereas a negatively charged atom is called a anion.
CRB True or false? A formerly neutral Carbon-14 undergoes beta-decay to become the anion Nitrogen-14.
False. A formerly neutral Carbon-14 undergoes beta-decay to become the CATION Nitrogen-14.
CRB Which of the following is an anion? (see periodic table here: https://www.ptable.com/Images/periodic%20table.png)
(A) Krypton-85 with 36 electrons
(B) Vanadium-45 with 21 electrons
(C) Cadmium-111 with 50 electrons
(D) Bromine-79 with 34 electrons
(C) Cadmium-111 with 50 electrons
Cadmium has 48 protons, so (C) is an anion.
Krypton has 36 protons, so (A) is neutral.
Vanadium has 23 protons, so (B) is a cation.
Bromine has 35 protons, so (D) is a cation.
CRB Which type of particles are most often involved in creating bonds between multiple atoms, since they have the weakest interactions with the nucleus?
(A) 1s electrons
(B) Neutrons
(C) Valence electrons
(D) Positrons
(C) Valence electrons
Because valence electrons are furthest from the nucleus, they have the weakest interactions with the nucleus, the strongest interactions with the environment, and they often form bonds between atoms.
Explain the term "half-life" and the shape of a half-life plot.
The half-life of a sample is how long it takes for half of the sample to decay. A half-life plot is a hyperbolic curve with an asymptote at y=0.
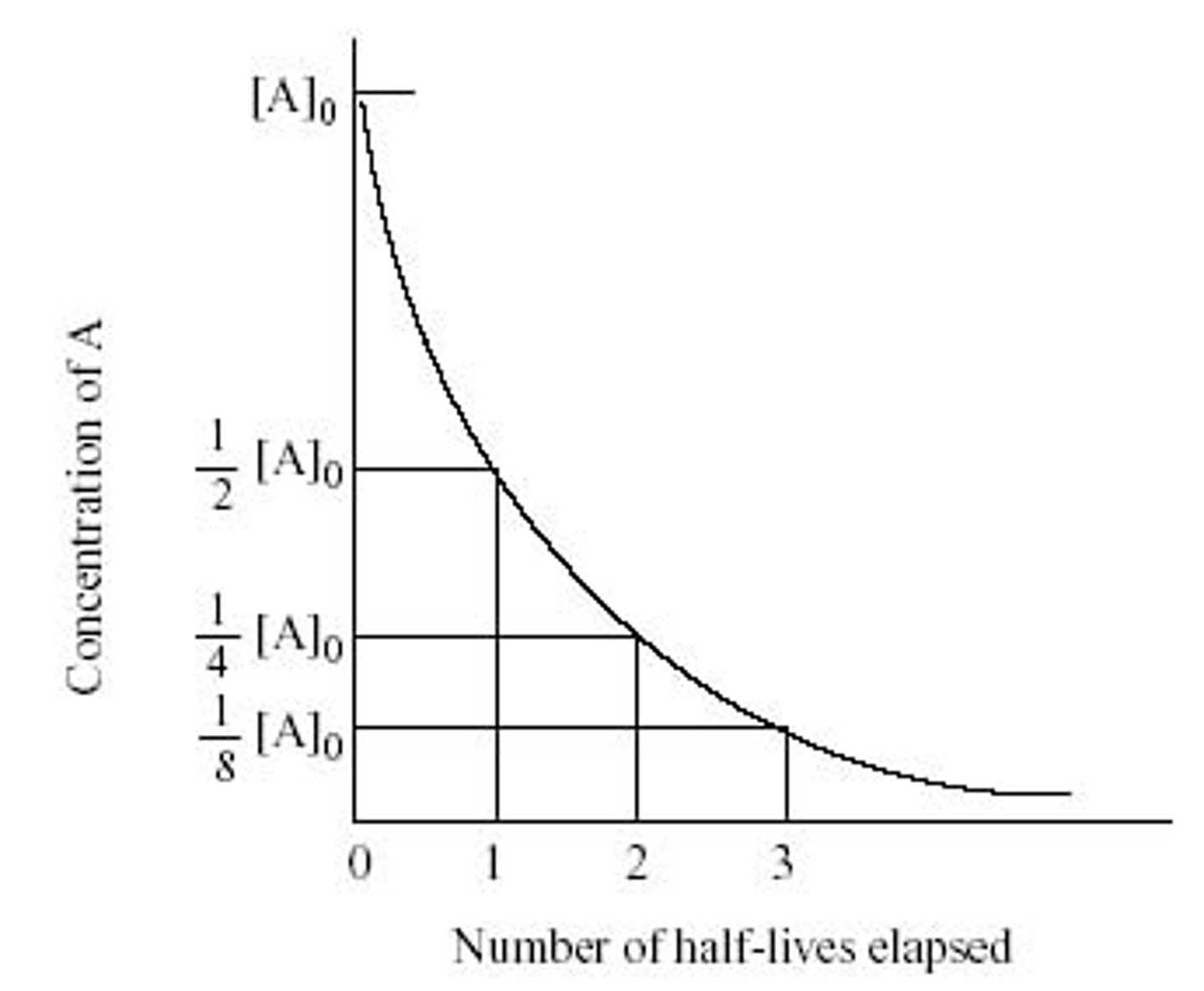
The half-life of Carbon 14 is 5,740 years. If you have 37 grams of Carbon-14, approximately how much Carbon will be left after 14,304 years?
(A) 4.875 grams
(B) 6.73 grams
(C) 10.43 grams
(D) 17.34 grams
(B) 6.73 grams
Approximation can be used to quickly find this answer.
(0 half-lives) At 0 years -> 37 grams
(1 half-lives) After 5,740 years -> 18.5 grams
(2 half-lives) After 11,480 years -> 9.25 grams
(3 half-lives) After 17,220 years -> 4.625 grams
Since 14,304 years is about 2.5 half-lives, the best answer choice is (B) 6.73 grams.
Need help with MCAT math? Become an MCAT math wizard using Andrew's High-speed Math Mastery Course @ https://mcatselfprep.com/course/andrews-high-speed-math-mastery-course/