Topic 17.4.1 Benzene
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What are aliphatic compounds?
Straight, branched or non-aromatic chains
What are aromatic compounds?
compounds that contain a benzene ring
What is benzene?
Benzene is an arene. Arene are unsaturated rings. Benzene has the formula C6H6. It is a colourless liquid with a boiling point of 80°C. It is found in crude oil and is a component of petrol. Benzene is a carcinogen.
Structure of Benzene
Benzene consists of a ring of 6 carbon molecules. Each of these carbon molecules are bonded to each other by single bonds. This leaves free electrons in p-orbitals. The p-orbitals overlap, creating pi-bonds and a region of electron density both above and below the carbon ring.
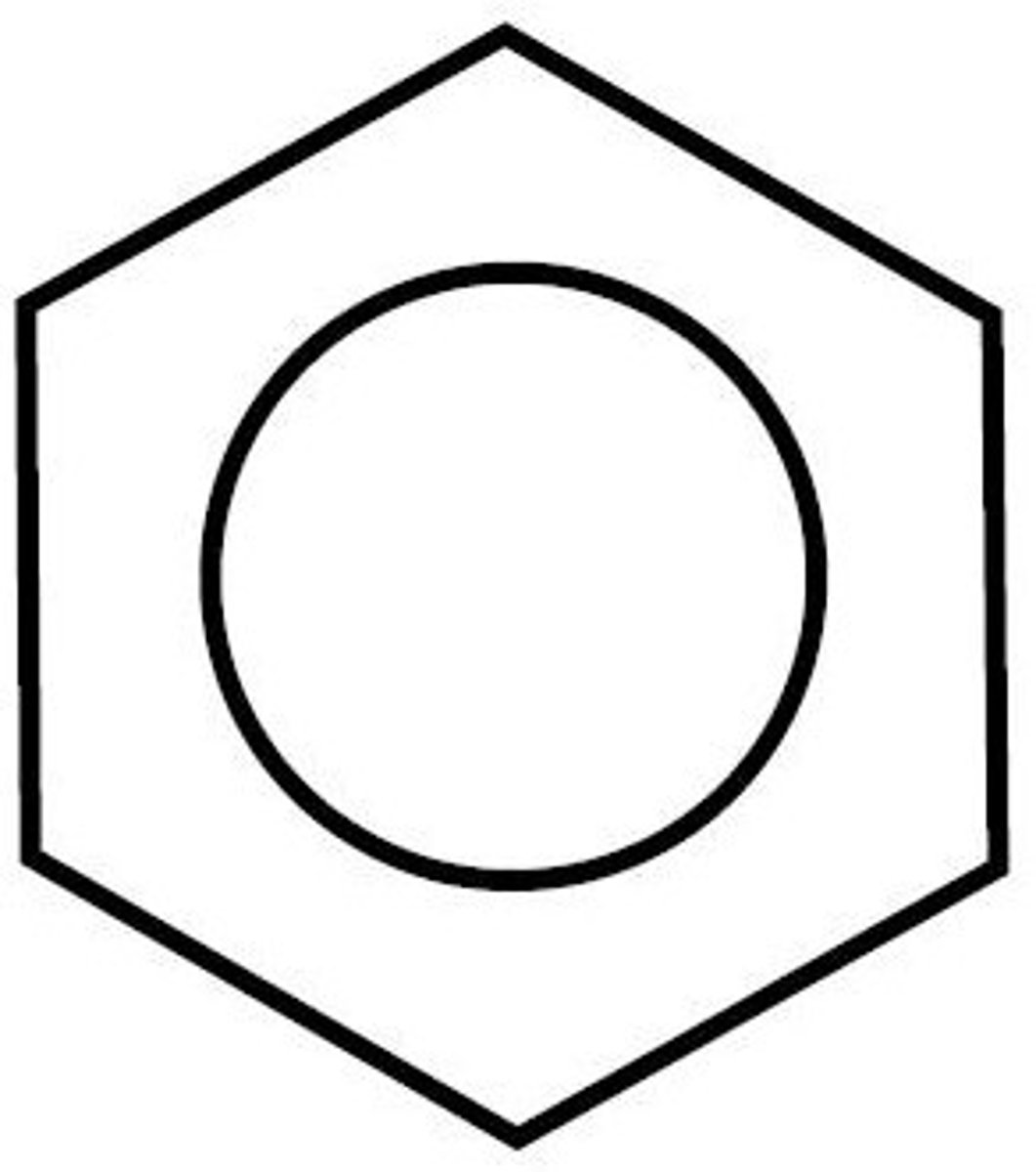
Kekule's structure
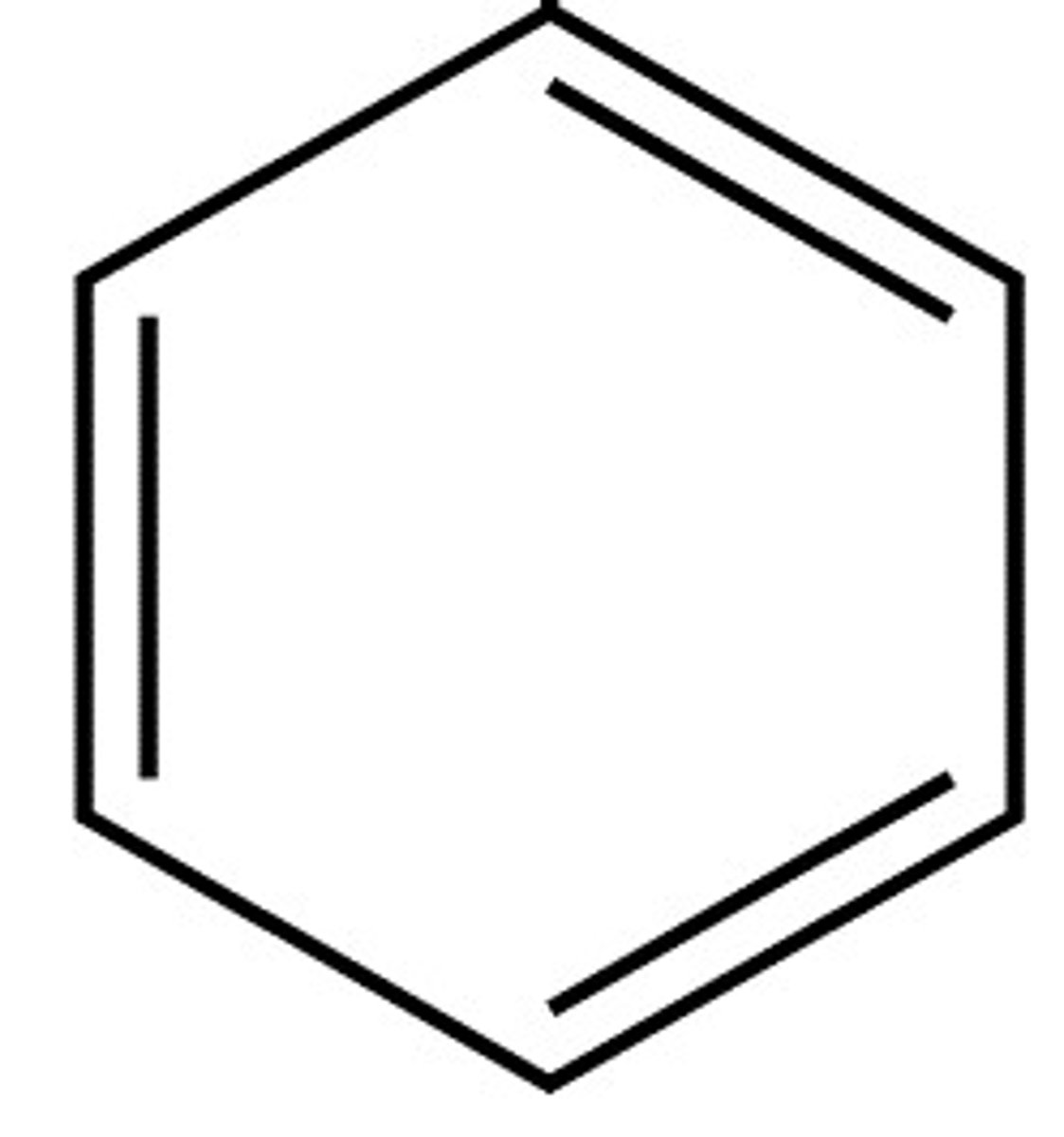
What are the problems of Kekule's structure?
1) If Benzene really has C=C bonds then it should readily decolourise bromine water, but it doesn't. When bromine does react with Benzene, a substitution reaction takes place. This evidence shows that there are no C=C bonds.
2) If Kekule's structure is correct, you would expect there to be 4 isomers of dibromobenzene but there are only three known to exist. This is because two of the isomers have the same infrared absorption, when according to the structure, they shouldn't.
3) ask Abdullah bhai
4) Kekule's structure could be named cyclohexa-1,3,5-triene, for which the value should be treble that of cyclohexane since Kekule's structure has three C=C bonds which would have an enthalpy change of hydrogenation value of -360, but the actual benzene has a value that is much lower.
Why does Benzene undergo a substitution reaction with bromine rather then an addition reaction?
Benzene undergoes a substitution reaction with bromine to form C6H5Br rather than addition to form C6H4Br is because substitution preserves the stability of the delocalised electrons in the pi bond. The addition reaction would instead produce a compound with two C=C double bonds, and that would lack the extra stability of the product of the substitution reaction.
Friedal-Crafts reaction
The two types of Friedal-Crafts reactions are: alkylation and acylation. They both have features in common:
- Using a reagent represented by XY, one of the hydrogen atoms in Benzene is substituted by Y, and the other product is HX.
- A catalyst is needed - often aluminium chloride, although other halogen carriers such as iron (III) chloride and iron (III) bromide also work
- Anhydrous conditions are needed because water would react with the catalyst and sometimes also with the organic product.
Naming aromatic compounds
Phenyl is used to represent the benzene ring when it is attached to another functional group.
What do all the electrophilic substitutions of benzene have in common?
- The benzene ring is electron rich. Although it is electrically neutral, the delocalised electrons in the pi bond above and below the atoms mean that the molecule attracts electrophiles.
- (Y+ = electrophile). As Y+ approaches the delocalised pi-bond, it attracts two of the six electrons, which form a covalent bond with it. This gives an intermediate species with a positive charge (shown by convention in the centre of the hexagon), in which one carbon atom is joined to both Y and H. There are now only four delocalised electrons, and these are represented by two thirds of a circle.
- This intermediate species lacks stability of an aromatic compound, so in the next step the H leaves as H+ and the two electrons in the C-H bond join the remaining four to restore the delocalised pi bond.
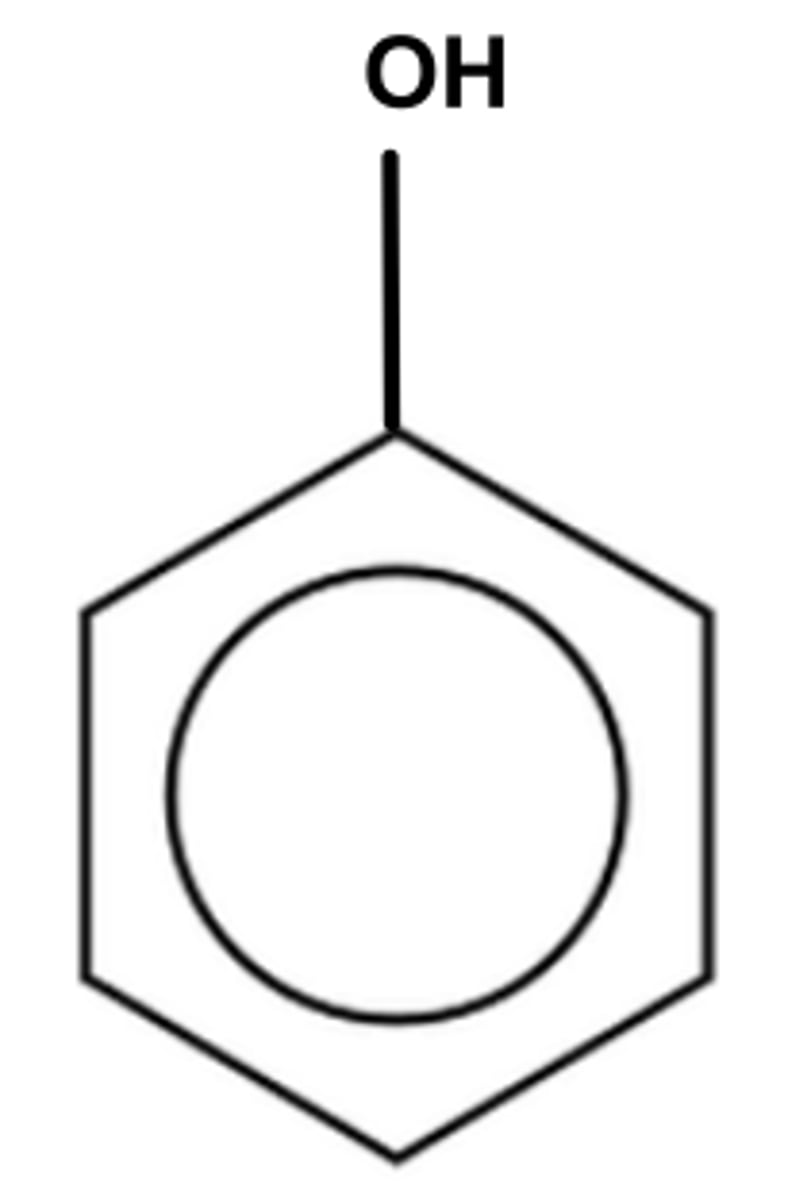
What is a phenol?
a hydroxy group/ alcohol bonded to a benzene ring
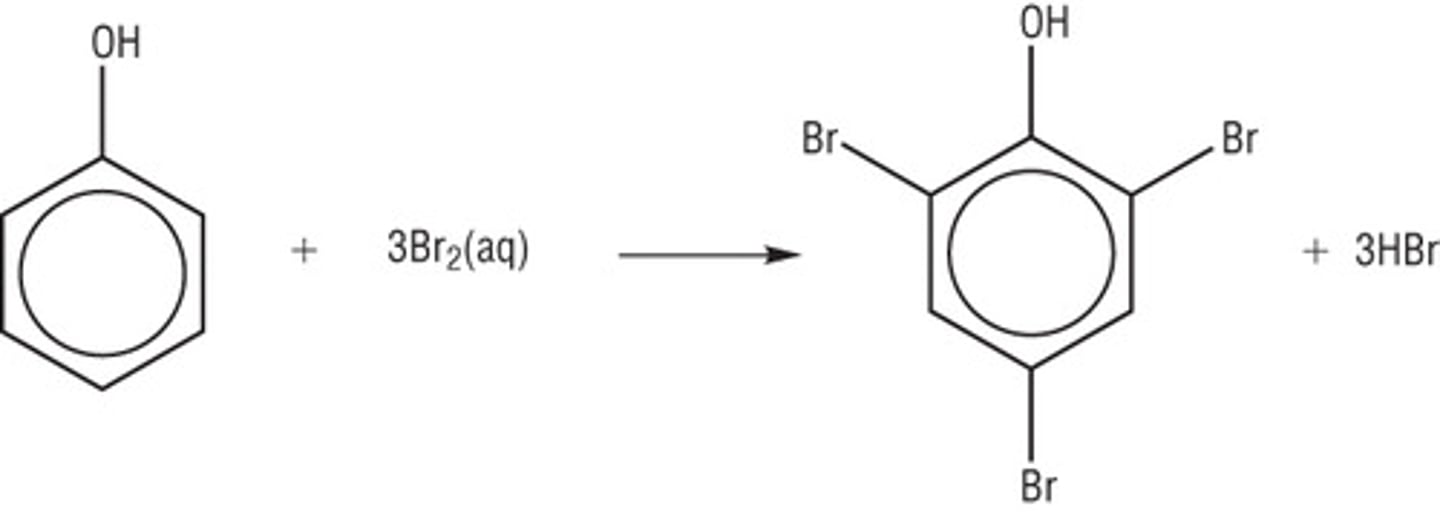
Bromination of phenol
Phenol + bromine water -> white precipitate of 2,4,6-tribromophenol
-Decolourises bromine water
-Halogen carrier catalyst not required
-Room temperature
Why does substitution happen much more readily with Phenol than Benzene?
- The oxygen in the OH group has lone pairs of electrons, and these electrons can merge with the electrons in the delocalised pi bond.
- The electron density above and below the ring of atoms is increased, a process sometimes referred to as 'activation' because the molecule is now much more reactive towards electrophiles.
- Bromine molecules, although originally non-polar, are polarised as they approach the benzene ring. Eventually, the Br-Br bond breaks and the Br+ electrophile attacks the benzene ring.
Uses of phenol
- Antiseptics
- Detergents
- Pharmaceuticals
- Dyes