H Chemistry - Unit 2 - 2i - Skincare
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
What is ultraviolet (UV) radiation?
Ultraviolet (UV) radiation is a high energy form of light, present in sunlight.
What can UV light do?
UV light can provide sufficient energy to break bonds within molecules. This causes sunburn and accelerates ageing of the skin.
What does sunblock do?
Sunblock products prevent UV light reaching the skin.
What happens when UV light breaks bonds?
When UV light breaks bonds, free radicals are formed.
What are free radicals?
Free radicals are atoms or molecules that are highly reactive due to the presence of unpaired electrons.
What are the different steps of free radical chain reactions?
Free radical chain reactions include the following steps;
Initiation
Propagation
Termination
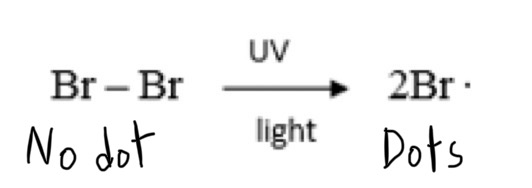
What is initiation?
Initiation is the formation of free radicals
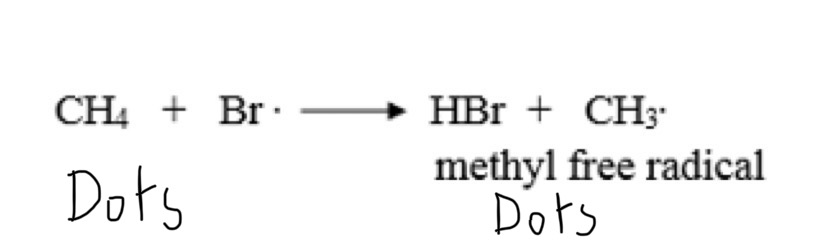
What is propagation?
Propagation is when free radicals stabilise by forming a neutral molecule and produce a second free radical. This causes a chain reaction that continues until no more reactants are left or termination takes place.
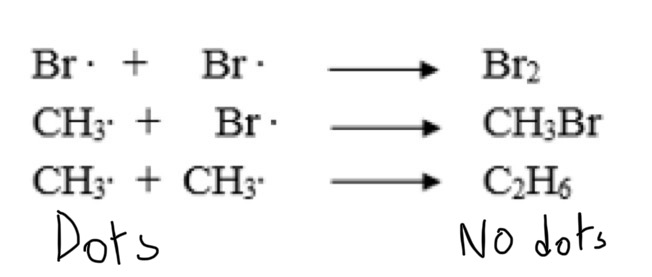
What is termination?
Termination is when two free radicals combine together
What are free radical scavengers?
Free radical scavengers are molecules that react with free radicals to form stable molecules and prevent chain reactions from occurring.
What are free radical scavengers added to?
Free radical scavengers are added to many products including cosmetics, food products, and plastics.