Alkenes
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
What is the reaction mechanism to make alkenes into haloalkanes
Electrophilic addition
Test for an alkene
Add to substance to bromine water, the solution will turn from brown to colourless
Conditions for hydrogenation
Nickel catalyst
423K
Markonnikoff rule
In electrophilic addition, the hydrogen in a hydrogen halide is more likely to join a carbon with the most hydrogen already attached
Why can electrophilic addition start
because the pi bond in an alkene is an area of high electron density so its easier to attract those electrons
What is the nature of a double bond
There is a pi bond between 2 p orbitals above and below the sigma bond between the s orbitals
can the double bond rotate
no
stereoisomerism definition
compounds with the same structural formula but different arrangement in space
what is the difference between an E isomer and Z isomer
Z isomer have their high priority groups on the zame zide and E have them on opposite sides
what are cis trans isomers
z and e isomers that have at least 1 hydrogen
what determines a high priority group when naming e/z isomers
when the next atom on the chain has a higher atom number
how can electrophilic addition occur to a non polar molecule
the negative region of a pi bond repels the shared pair of electrons, making the atom near the atom positive
What is a pi bond
The side to side overlap of p orbitals
What properties does a pi bond cause in alkenes
Not able to rotate about the double bond
Electrophiles can react with the double bond
High bond enthalpy
What are the conditions for an alkene to become an alcohol using electrophilic addition
Acid catalyst (H3PO4) and steam
What must a repeat unit have
n in bottom right, lines must come out of the square brackets
Polymerisation reaction equation for (poly)ethene
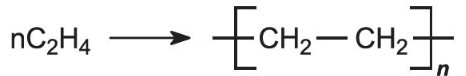
Which carbocation is most stable
Tertiary
What happens when plastic is recycled
It is cut up and reduced to pellets which can be used again for certain structures
Why can PVC not be burnt
Releases, as well as CO2, hydrogen chloride
What is an advantage of feedstock recycling
Can use unprocessed polymers
What are biodegradable polymers usually made up of
Natural materials like starch and cellulose
What are photodegradable polymers
Plants that break down in the sunlight
What is feedstock recycling
Processes that reclaim monomers, gases and oil and waste