14/15/16/17) Zoning, Crystallography, Mineral Description, Symmetry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
what is zoning?
compositional variations within a single grain. Edges are dark and get lighter in the center, variation from core to rim, zoning developed in formation of the rock, compositional zoning can develop in minerals that exhibit solid solution
explain plagioclase zoning
igneous rock, oscillatory zoning (repeated changes in composition of mineral), patchy zonation, irregular compositional patches
Is quartz’s undulatory extinction caused by zoning?
no its caused by deformation
explain uranium dating with zircon
zoning formed from variation in uranium content, only happens from uranium levels, very long term growth stages (instead of less than a year like plagioclase)
Explain plagioclase solid solution
Na and Ca endmemebrs, albite and anorthite
explain plagioclase phase diagram
Liquidus: everything above is melted and stable, upper limit.
Solidus: solid solution, everything below is stable and solid
Middle area: solid plagioclase and melt liquid exist
Solid solution will start melting at the solidus and finish melting at the liquidus.
The line at the bottom is %Anand %Ab composition of plagioclase.
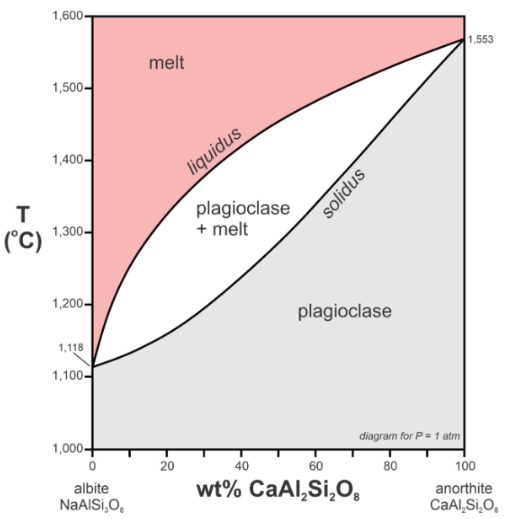
Explain the tie line
connects the melt in eqlm with the solid at the same temperature. A melt with originally 70% An will start crystallizing with crystals that are 90% An. The crystals at B are at eqlm with the temp at A, it’s no longer at eqlm, the early formed crystals will react with the melt. Crystals maintain eqlm with the melt as it cools down.
Keep going to point E, the crystals formed are even more Na rich, at which point the tie line reaches point F, which is the same composition as the starting melt. After this, no more compositional changes will occur, just cools down normally.
Summary: C will crystallize with composition D, AND B will react with the melt to become more albite rich so that eqlm is maintained as crystallization proceeds.
How does zoning happen in terms of phase diagram?
you somehow prevent the solid at B from reacting with the melt and keeping eqlm. This causes zoning because there will be diff compositions of the mineral in diff areas
Non-eqlm zoning
early formed crystals did not change composition due to diseqlm
Ant rich core, Alb rich rim = normal
fresh batch of magma comes in to add on to the crystal, repeated injections of fresh magma, possibly calcite rich which would go through the zoning process again
difference between zoning in olivine and zoning in plagioclase
zoning in plagioclase is common because Na and Ca are big and it’s hard for them to move through and around.
zoning in olivine is rarer because Mg and Fe are smaller and they can move around easily, making it harder for zoning to occur
explain alkali-feldspar incomplete solid solution phase diagram
Na and K are different sizes so they don’t have a complete solid solution
Double curve because you can’t replace all Na with K because K is bigger than Na.
Substitution happens at very high temps because it’s hard to substitute differently sized elements, it’s more flexible at high temp
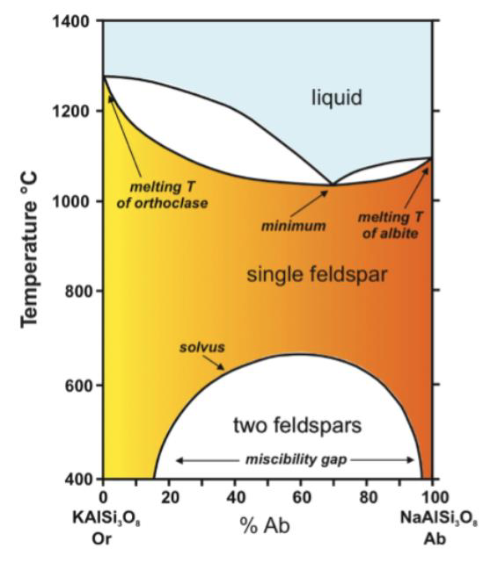
explain alkali feldspar exsolution
exsolution lamellae of albite
perthite: host of K-feldspar and lamellae of Na feldspar
perthite is more common in igneous, anti-perth rarer but more common in metamorphic
exsolution is crystallographically controlled
tartan twinning (perthite) is characteristic of k-feldspar
multiple albite twinning (anti-perth) is characteristic of plagioclase
what are the crystal systems
isometric, monoclinic, triclinic, orthorhoombic, tetragonal, trigonal, hexagonal
what are miller indices? hexagonal
notation to quantify planes in a crystal, hkl, use {} for repeating planes/cleavage, [] for direction/axis
hkil, a3 is negative
summary of things in mineral description
formula, crystal system, HM notation, axis length, Z=formulaic units in a single unit cell, H=hardness, G=specific gravity, optic sign, refractive indices, birefringence, 2V angle
what are the symmetry elements
mirror plane, axis of rotation, inversion center
explain axis of rotation, examples
how many times do you see an identical face on 360° rotation, orthorhombic crystal = three 2 fold, isometric: abc=4fold, corners=3 fold, edges=2 fold
explain inversion center
center of symmetry through which you can invert the crystal, if there is a corresponding face on the opposite side for all faces it has center of symmetry
what is roto-inversion, examples?
combining rotation and inversion. Bar 1 axis: rotate 360 then invert = same as center of symmetry. Bar 2 axis: rotate 180 then invert = same as mirror plane. Bar 4 axis: rotate 90 then invert = 2 fold rotation axis. Bar 6 axis: rotate 60 then invert = 3 fold rotation axis and mirror plane between faces
what’s special about a 3fold rotation axis?
if you have a center of symmetry and do a 3fold roto-inversion you get the same face