Pchem Exam 3 (spectroscopy and PK)
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
what do spectroscopic techniques provide
snapshots of a molecule as different types of energy affect a molecule differently
IR uses
provides a method to determine the presence of functional groups
how IR works? (what are atoms constantly doing and what does IR do to them)
atoms are in constant motion and these movements are called vibrations. IR radiations has just the right amount of energy to stretch or bend a bond (but not break it)
this results in the molecule absorbing energy resulting in an absorption band at that frequency (so all bonds have a frequency range where they will absorb IR energy)
type of movement for molecules in IR (6)
symmetric stretching, asymmetric stretching, wagging, twisting, rocking, and scissoring 😜
where do most organic molecules absorb energy (prob don’t need to know)
the mid infrared region (4000 cm-1 - 6000 cm-1)
what does an OH group look like on IR
broad band and on the left side of the graph
IR NH2 how does it look
has two fangs
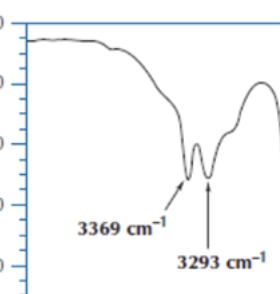
what units are IR energy represented in and which is the more common one for us
wavenumber (what we use) and wavelength
relationship between wavenumber and wavelength
higher wavenumber correlates to a lower wavelength (which makes sense because a lower wavelength means higher energy)
atomic mass trend in IR
wavenumber (frequency) increases with increasing atomic mass
put these compounds in order of highest to lowest wavenumber
N-H, C-H, O-H
O-H > N-H > C-H (atomic mass trend)
bond order trend in IR
wavenumber (frequency) increases with increasing bond order
triple bond > double bond > single bond
how does NMR work? what happens to atoms?
excites protons so that their spins align against a magnetic field
the output is a range of frequencies that correspond to the type of proton
NMR uses
determine exact structure of compound and impurities
NMR shift; what does it tell us abt a proton
tells us what type of proton we have. strongly influenced by the surrounding electron density (electronegativity)
where is downfield on NMR and what does it mean for electronegativity
to the left; higher ppm; proton next to a more electronegative atom
where is upfield on NMR and what does it mean for electronegativity
to the right; lower ppm; proton next to a less electronegative atom
NMR integral; what does it say abt the proton
how many protons in relation to other peaks (RATIO so 1:2:3 cld be CH, CH2, CH3 or CH2, CH4, CH6, etc)
NMR splitting; what does it say abt the proton
how many protons are next to the proton we are looking at
meaning of singlet, doublet, triple, quadruplet
H is next to 0 H’s, H is next to one H, H is next to two H’s (CH2,), H is next to 3 H’s (CH3,)
if your looking at the CH in CH2CHCH3 then its two diff types of protons on each end so wld be like doublet of triplet or triplet of doublet but he wld prob not ask that like 99% sure
when are protons equivalent and how does that look on NMR
when there is a plane of symmetry and the equivalent protons will show up as a single peak
what does it mean if there is a plane of symmetry and two H’s on the same C are still showing up as two different peaks
it means there is a double bond (OLEFIN) and double bonds are not rotatable so it means the H’s wouldn’t be equivalent
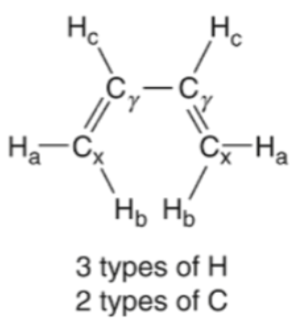
olefin
c--c DOUBLE bond
what can splitting in NMR be useful to find out
location of groups on an aromatic ring/cyclohexane ring
what does it mean if you see 4 signals in the aromatic region? how many substituents?
4 H’s , 2 substituents
NMR aromatic ring region
7-8 from class (6.5-9 in acc chart)
NMR x axis
ppm
UV (UV-vis) what do the compounds do when exposed to UV light
compounds absorb UV light thru excitation of electrons
what does a compound need to be UV active
accessible electrons; compounds with pi bonds
when is UV absorption more intense
conjugation; the more double bonds there are
what is absorbance intensity proportional to
concentration (amount of light absorbing substance (solute) in a solution (aka pi bonds))
beer’s law
A = Ebc
E= extinction coefficient (like a greek weird E)
b = path length
c= concentration (amount of light absorbing substance (solute) in a solution (aka pi bonds))
UV detection method (what enzyme wld u use)
ornithine aminotransferase
UV detection method (what compound wld u use and y)
NADH bc its UV active
how does UV detection work if you wanna see how active an enzyme is but its not UV active
ex) ornithine aminotransferase (O) (not UV active)
want to see how fast O is working but not UV active so u cant see under light
nadh is showed under UV so u use it
nadh reacts w O and the light keeps getting dimmer as NADH gets used and turns to NAD+ which means the O enzyme is working (active) to convert this
by measuring how much light nadh has over time you can see how active the enzyme (O) is
this method is used to see if the enzyme is working and at what rate it’s working
fast drop (steep) of nadh means enzyme is working rly fast rate and slow drop (not steep slope) shows enzyme working slow
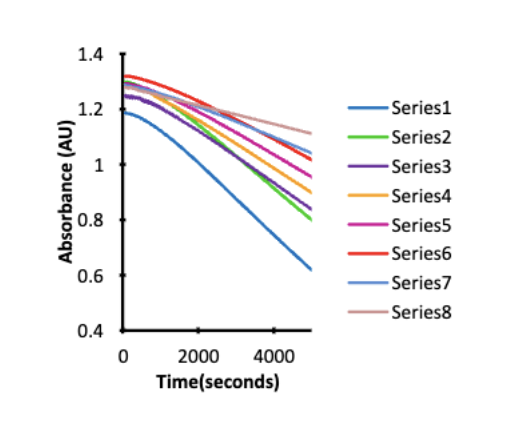
UV-vis units you measure and graph x/y axis
Absorbance (y axis) and wavelength (x axis/ nm)
fluorescence; how is it diff than UV-vis
certain molecules can be excited by UV/visible light BUT will emit radiation at a longer wavelength
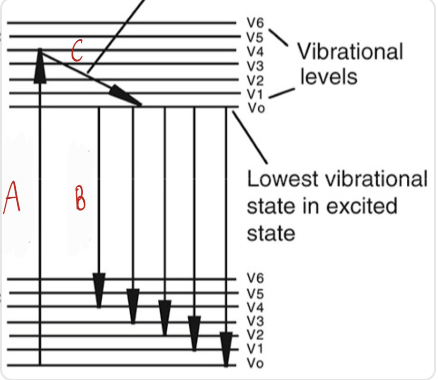
what is happening at A, B, C
A = excitation
B = relaxation
C = emission
between excitation, relaxation, and emission which are radioactive transitions
excitation and emission
relaxation is a non radioactive transition
how does excitation happen and what does it mean when a molecule is at a higher energy level
when UV or visible light shines on a molecule electrons get excited
if the molecule is at a higher excited energy level it got more/absorbed more light
what is relaxation
as its on a higher excited energy level for a while the molecule starts to loose energy and starts relaxing (slanted line)
no light is emitted and this is why molecules emit light at a higher wavelength (lower energy) bc some is lost during relaxation
what is emission
when the electrons fall back down to ground state
as they fall they release energy as light known as fluorescence
relationship between energy of the particles and wavelength
higher E means lower wavelength
If a molecule is excited at a wavelength of 340 will the emitted fluorescence be greater or less than 340
greater bc the emission will be a lower E bc some got lost in relaxation so the wavelength wld be like 500 nm
uses of fluorescence and when is a molecule fluorescent
to measure binding of a drug to a receptor/target, used for detecting fluorescent impurities
drugs are fluorescent when they have extended pi systems
is fluorescence predictable?
no
is quinine naturally florescent
yes bc it has an extended pi system
what molecules need to be cleaved to be fluorescent
sugars and peptides don’t allow a normally fluorescent molecule to be fluorescent so u cut them off with enzymes
what enzymes cleave the sugar and peptide bond
glycosidase and protease (cleave amide bonds between n and carbonyl c)
how can u measure an enzymes activity with cleaving in fluorescence
as you cleave the sugar or peptide bond that compound will start be be fluorescent
so you can measure the brightness of the compound and that will tell you the enzyme activity
more fluorescent means. more enzyme being used
why are we covering PK (MS, HPLC, like how all the stuff we did is not overlapping)
to identify to compound and find its concentration
radiometric assays (whaat do they do)
they take a small molecule and tag it w a radiolabel
to do this they replace for example a 12C with a 14C and then can follow the drug
common radio label substrates
3H, 14C, 32P
what is the radio labels detected by
scintillation counter or MS
they will detect the radio active molecule —> send signal —> results in peak
what do you usually need to do for radiometric assays
purification
methods of purification (2)
1) HPLC - as it comes off can detect if molecule is radioactive bc installed radio label on substrate (usually MS is done right after; HPLC-MS)
2) onbead purification
drawbacks to radiometric assays
special precautions needed for radioactive material
synthesis/ purchase of radiolabeled materials (expensive)
benefit of radiometric assay
selective (can detect between isotopes C12 vs C14)
traceable
what was radiometric assays replaced by
MS
PK graph x and y axis
x axis: time
y axis: amount of drug in blood
PK graph first half vs second half
first half: absorption
second half: elimination
PK graph what is the peak and what does it mean
Cmax, highest concentration of drug in blood
PK graph where is t1/2
in the middle (half the conc of Cmax then from there find the time)
PK graph what is the bottom point and what does it mean
Cmin: lowest concentration of drug in blood
what can PK graph show
look how much drug in blood, plasma, target, receptor