The Photoelectric Effect
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Photon
Packet of energy (a wave, yet acts almost like a particle)
What is the photoelectric effect?
If you shine radiation of high enough frequency onto the surface of a metal, it will instantly emit electrons.
Photoelectric effect diagram
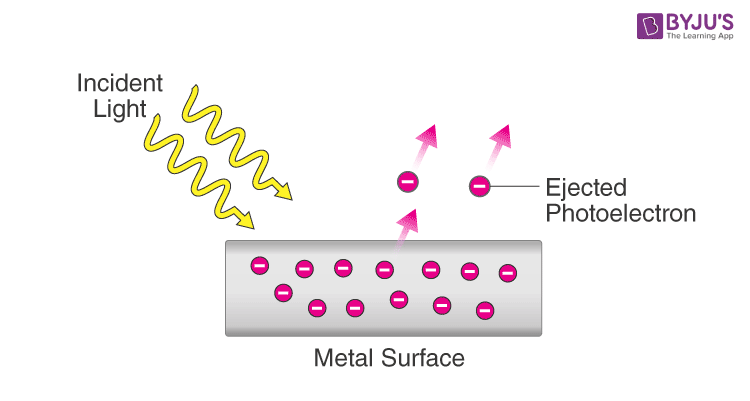
For each 1 photon incident on the metal surface, how many electrons are emitted? What is this interaction called?
1
one-to-one interaction
Work function φ
The minimum energy to release an electron from a metal surface
What will happen if the work function is greater than the energy of a photon?
No electrons will be released
Threshold frequency
The minimum frequency to release an electron from a metal surface
What is the difference between the work function and the threshold frequency?
Work function - metals
Threshold frequency - photons
Intensity
Number of photons / energy of photons
Unit for intensity
Jm-2s-2 = Wm-2
One-to-one interaction
One photon can release one electron
Current
Rate of flow of charge (electrons)
Monochromatic
All photons have the same λ/f/E
Describe what happens if an electron is not on the metal surface
Work is done (energy is used) to bring the electrons to the surface. Then the electron is released but with less Ek than those that were already on the surface, thus demonstrating the (max) in the equation [Ek(max)]
Stopping potential
The potential difference required to stop electrons flowing.
What is the stopping potential based on?
The kinetic energy. The stopping potential is basically just the Ek in eV.
Equation used for stopping potential
E = QV —> V = E/Q