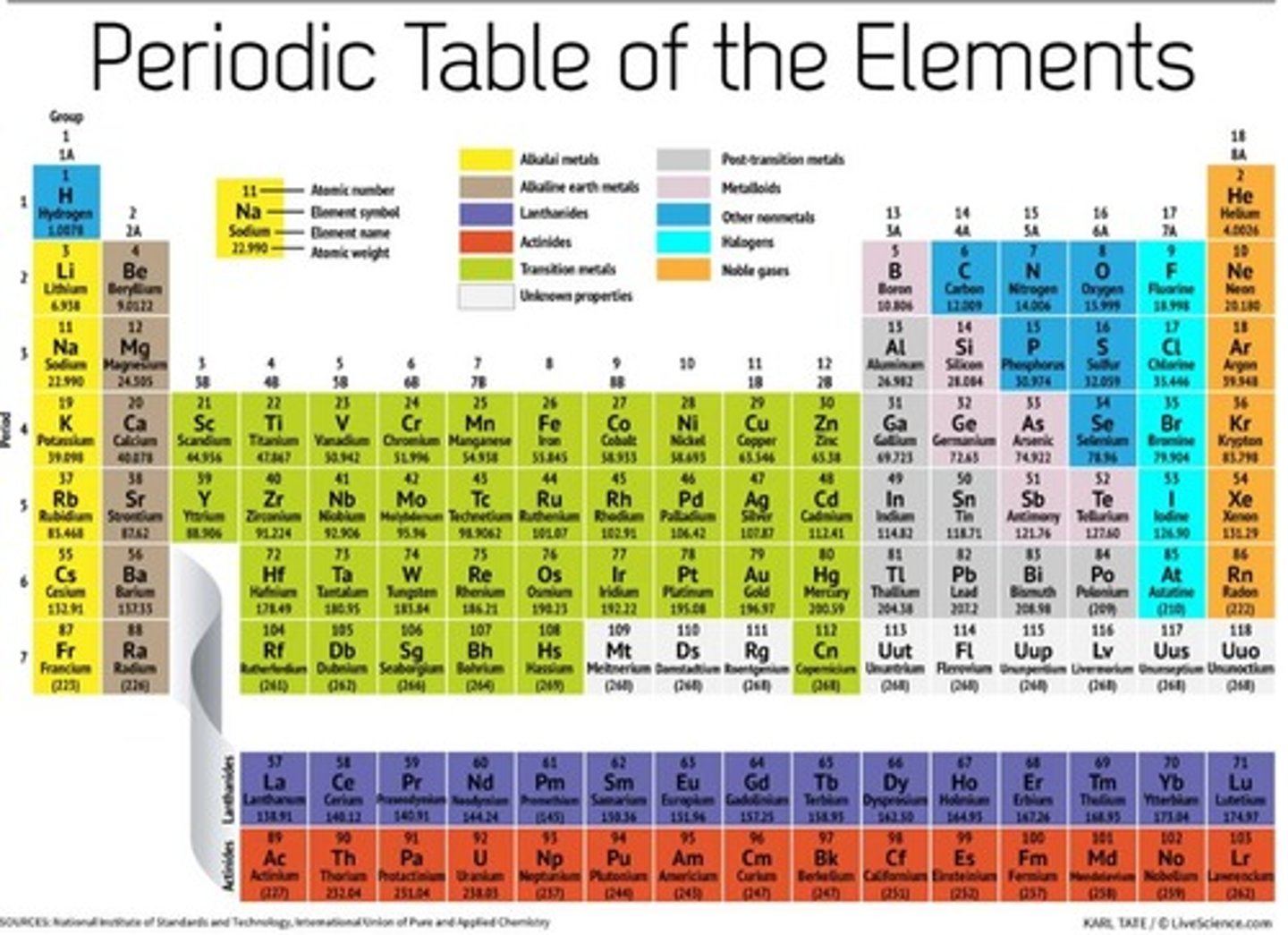
Periodic Table (The Periodic Table)
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
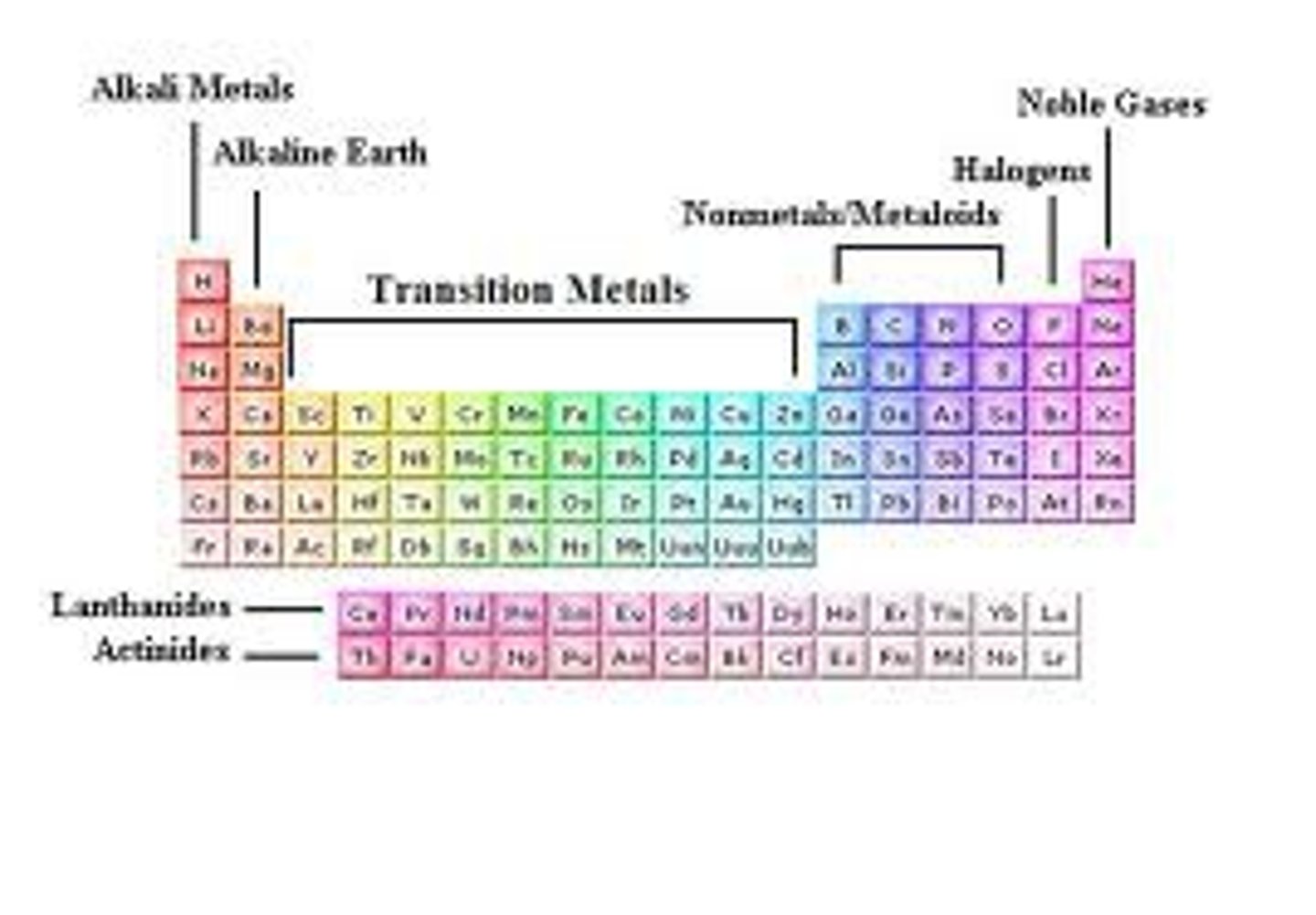
Group 1
Alkali metals.
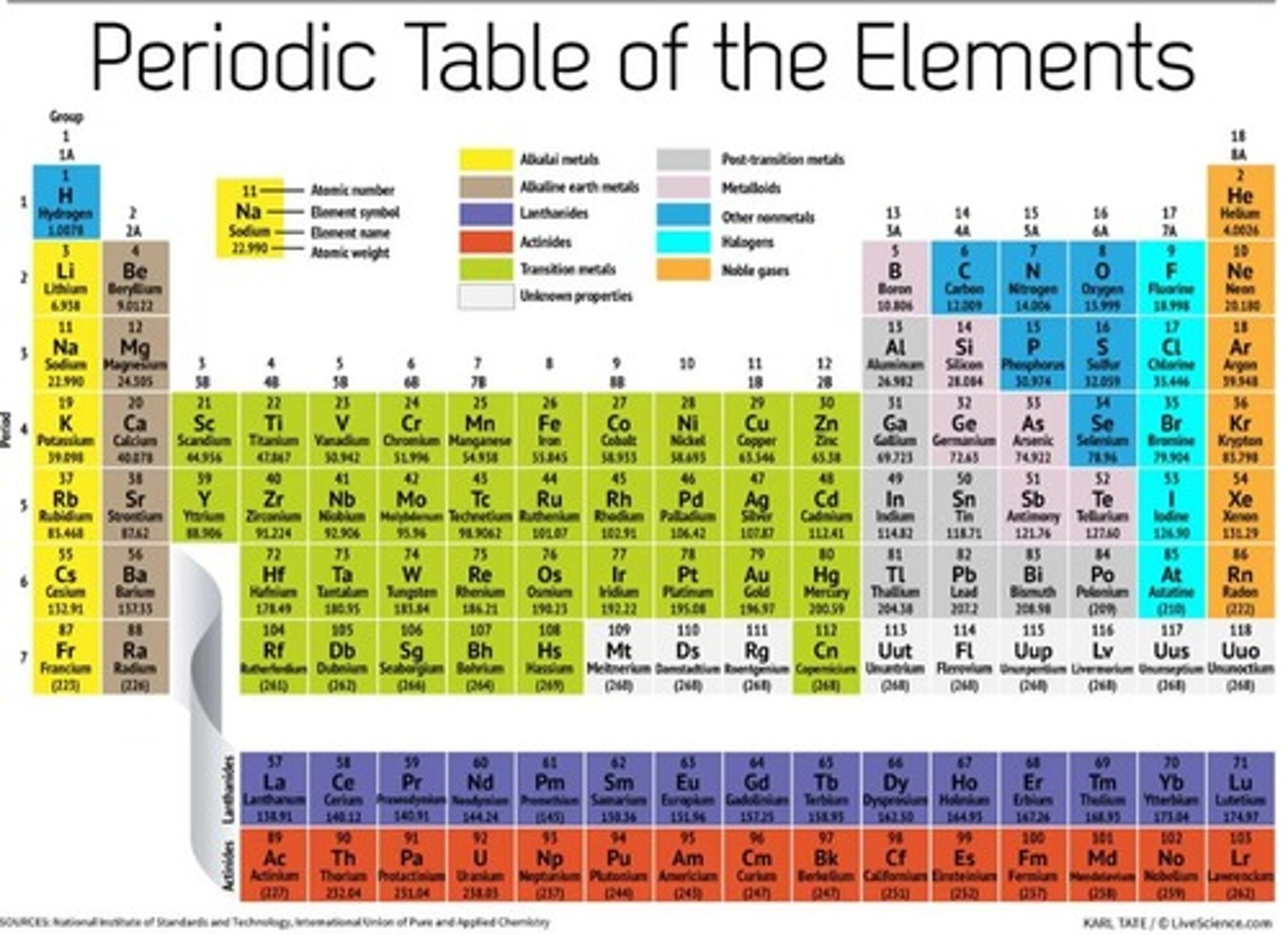
Group 2
Alkaline earth metals
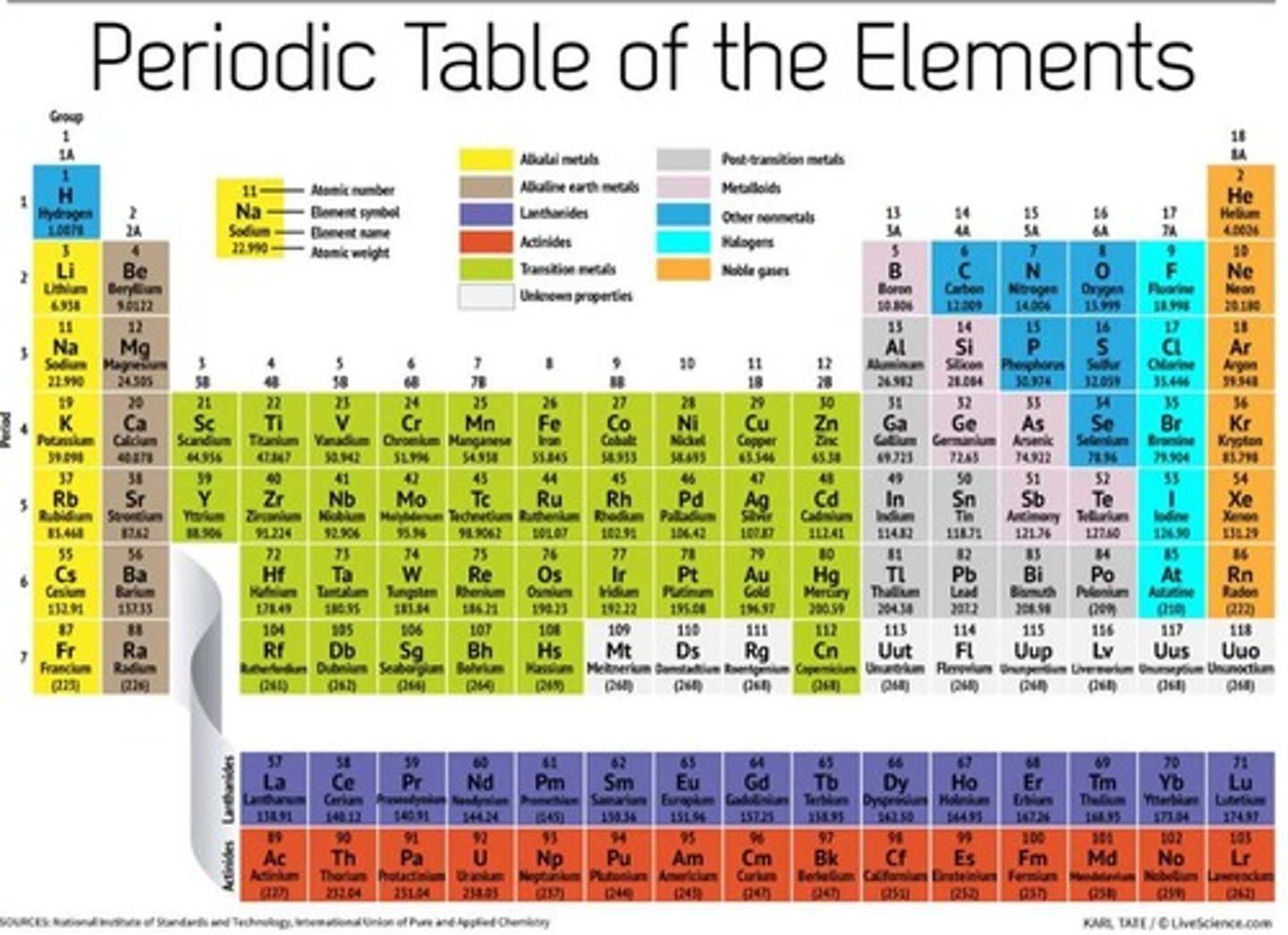
Groups 3-12
transition metals
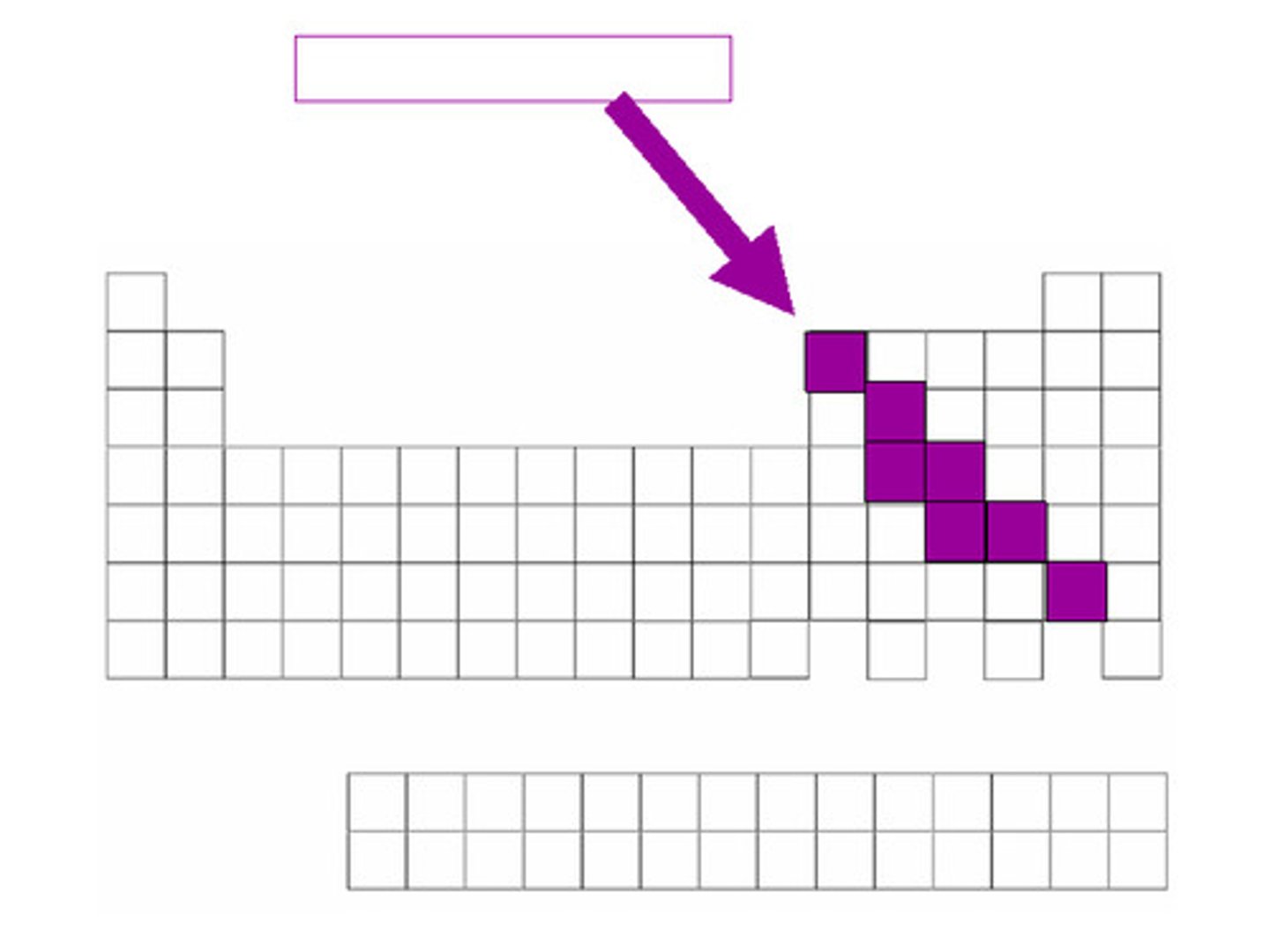
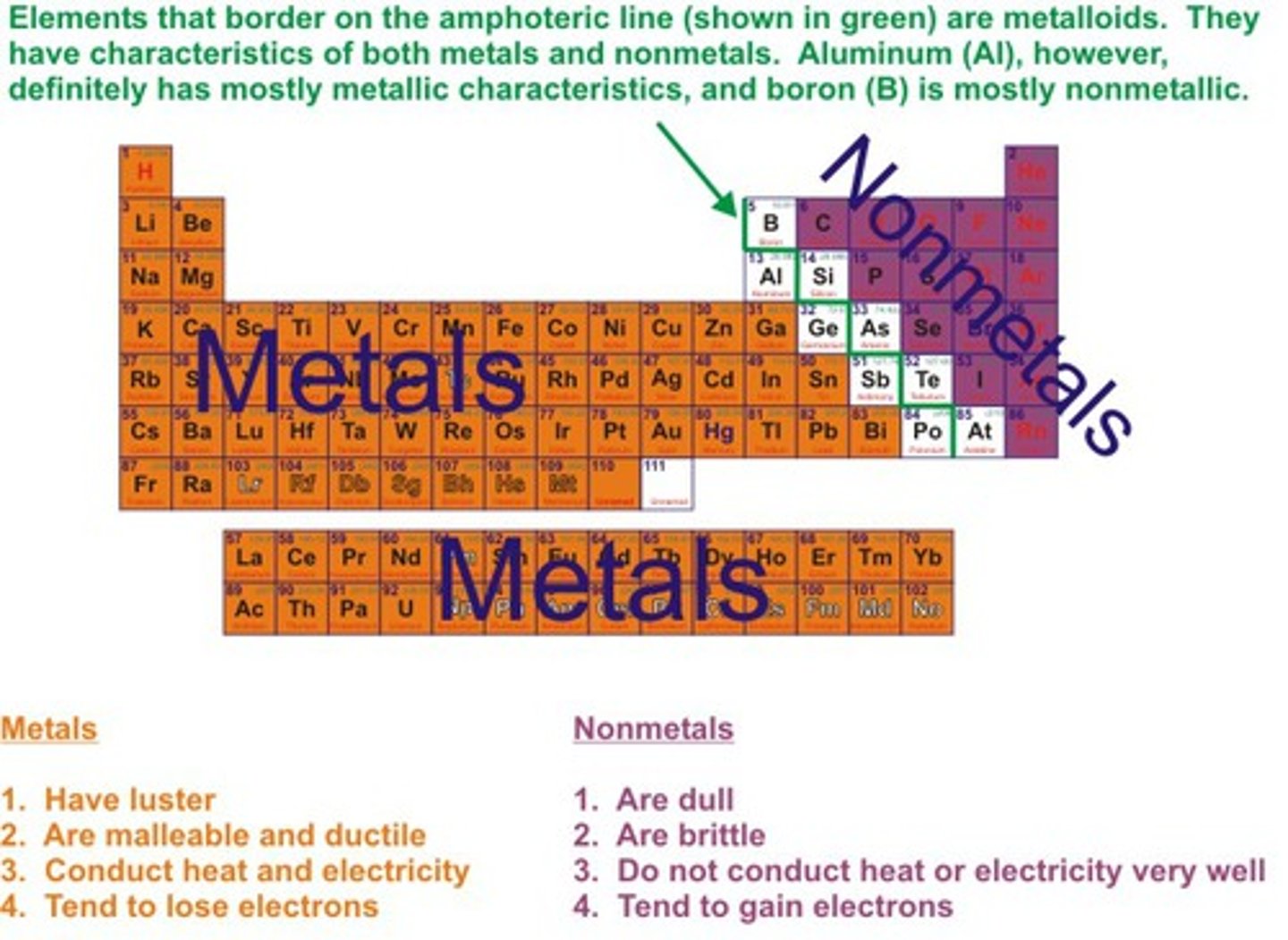
metalloids
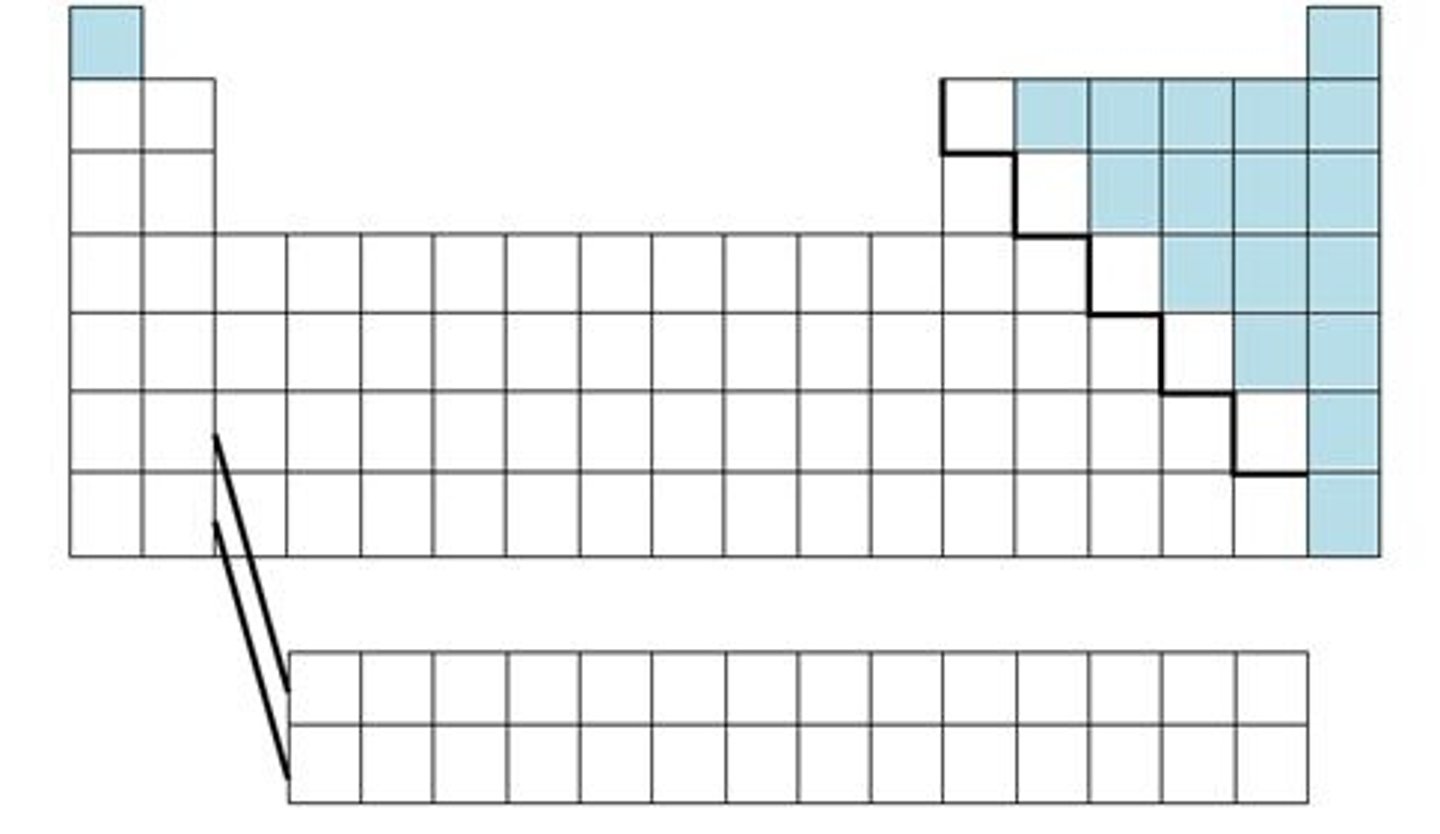
Found along the 'staircase'. Have properties of both metals and nonmetals
nonmetals
brittle , dull, poor conductors of heat and electricity
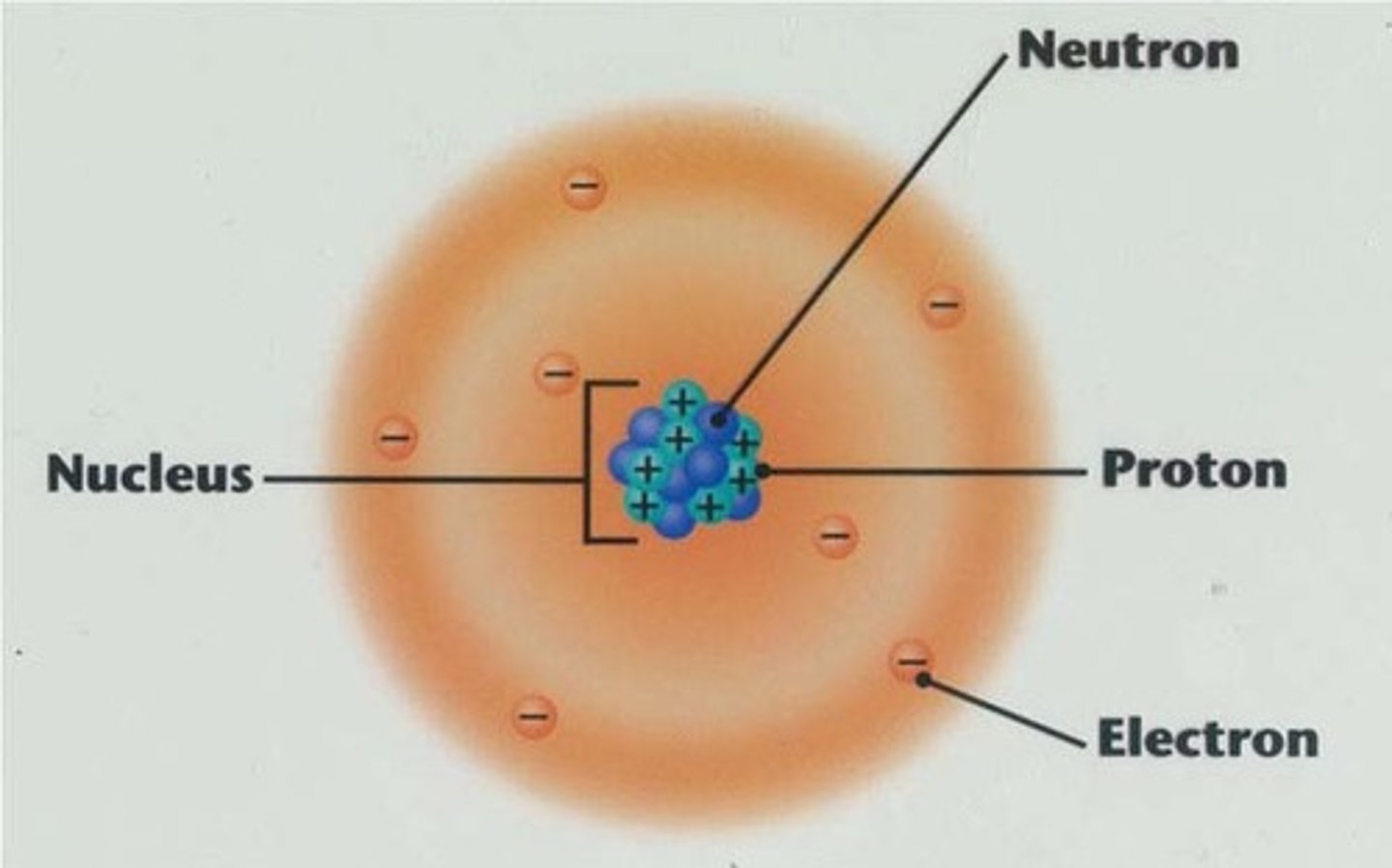
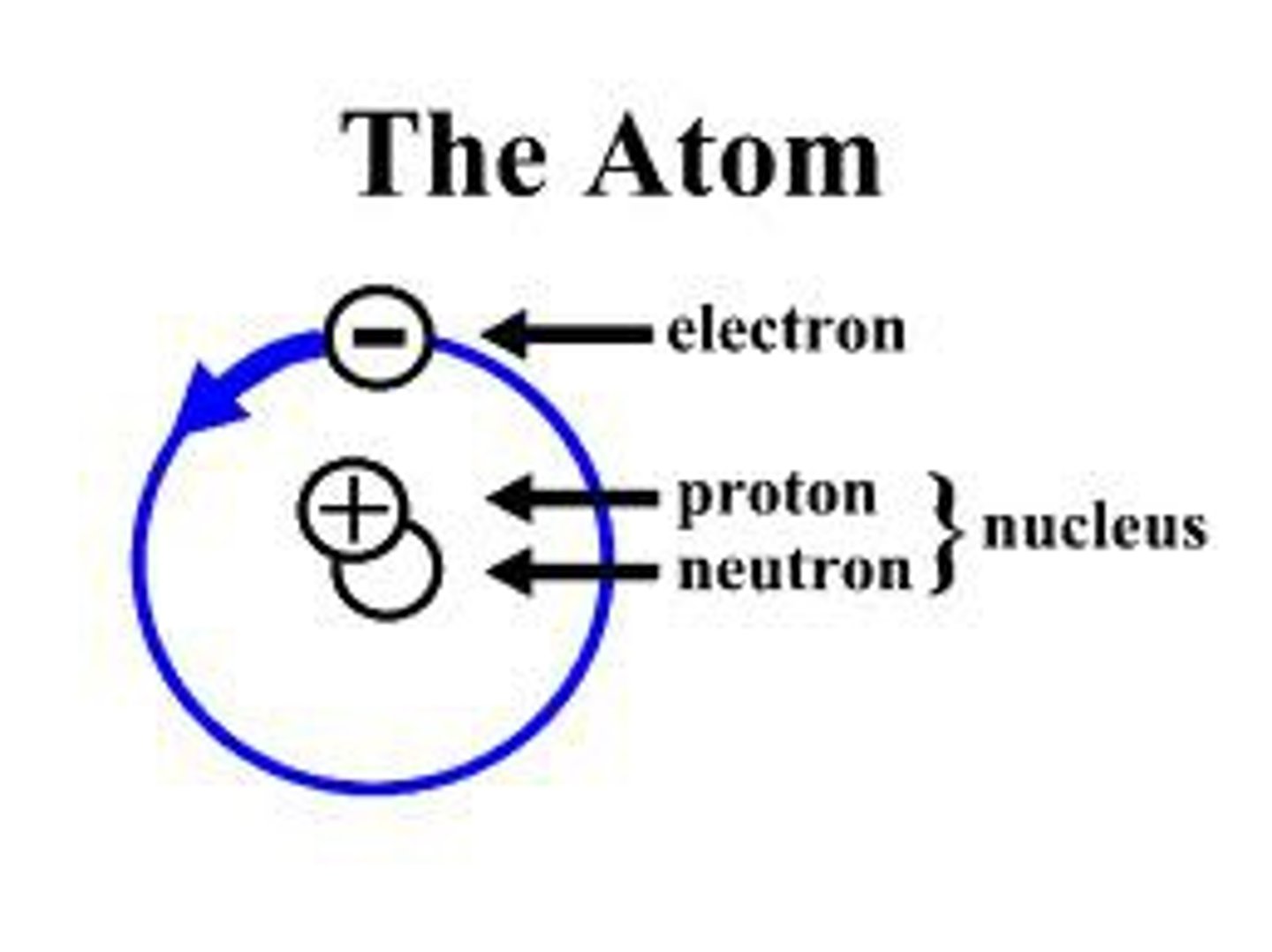
Proton
Positively charged particle in the nucleus of an atom (similar mass to neutron)

Neutron
A subatomic particle that is neutral and found in the nucleus of an atom (similar mass to proton)
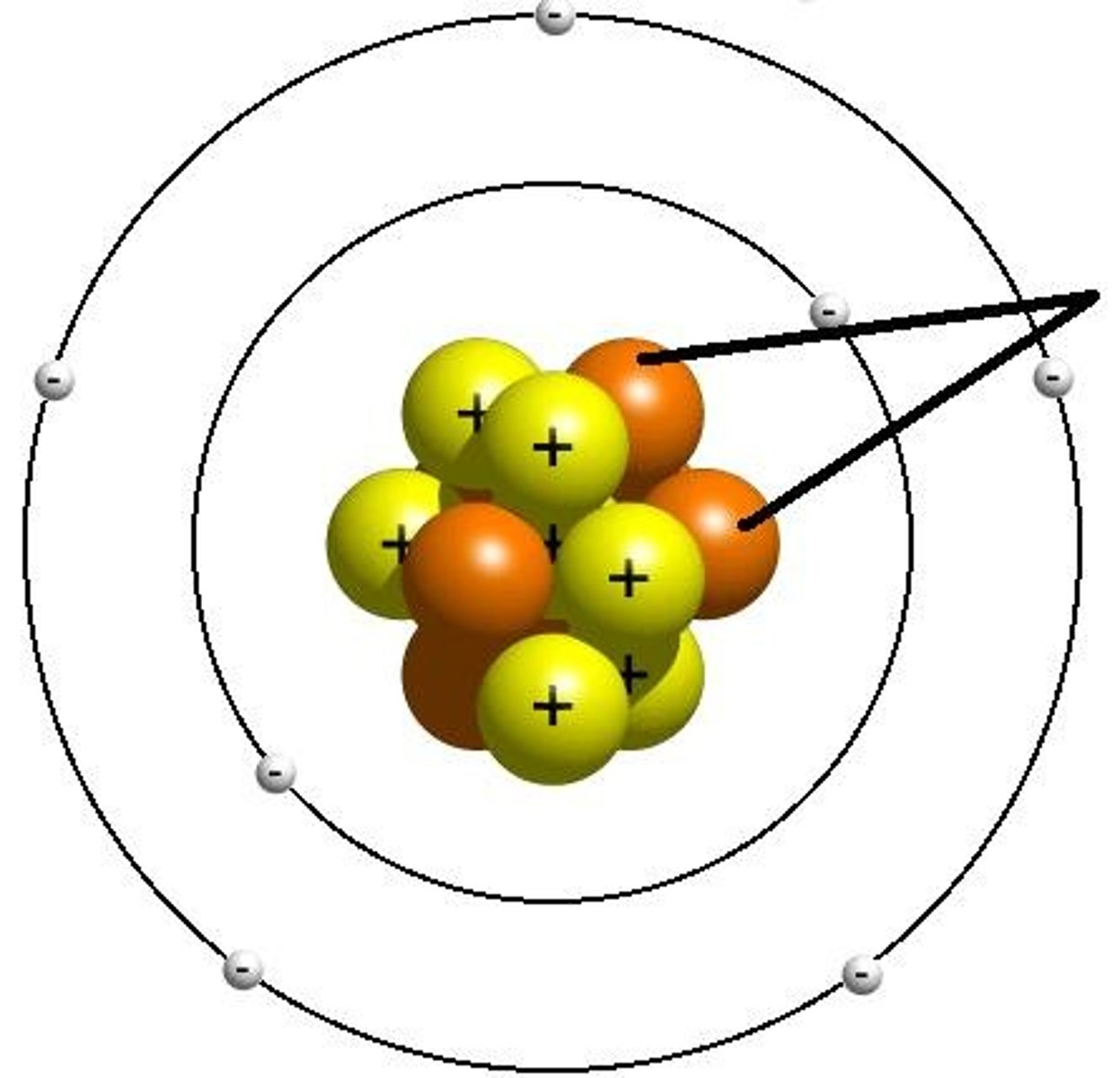
Electron
A tiny, negatively charged particle that moves around the nucleus of an atom.
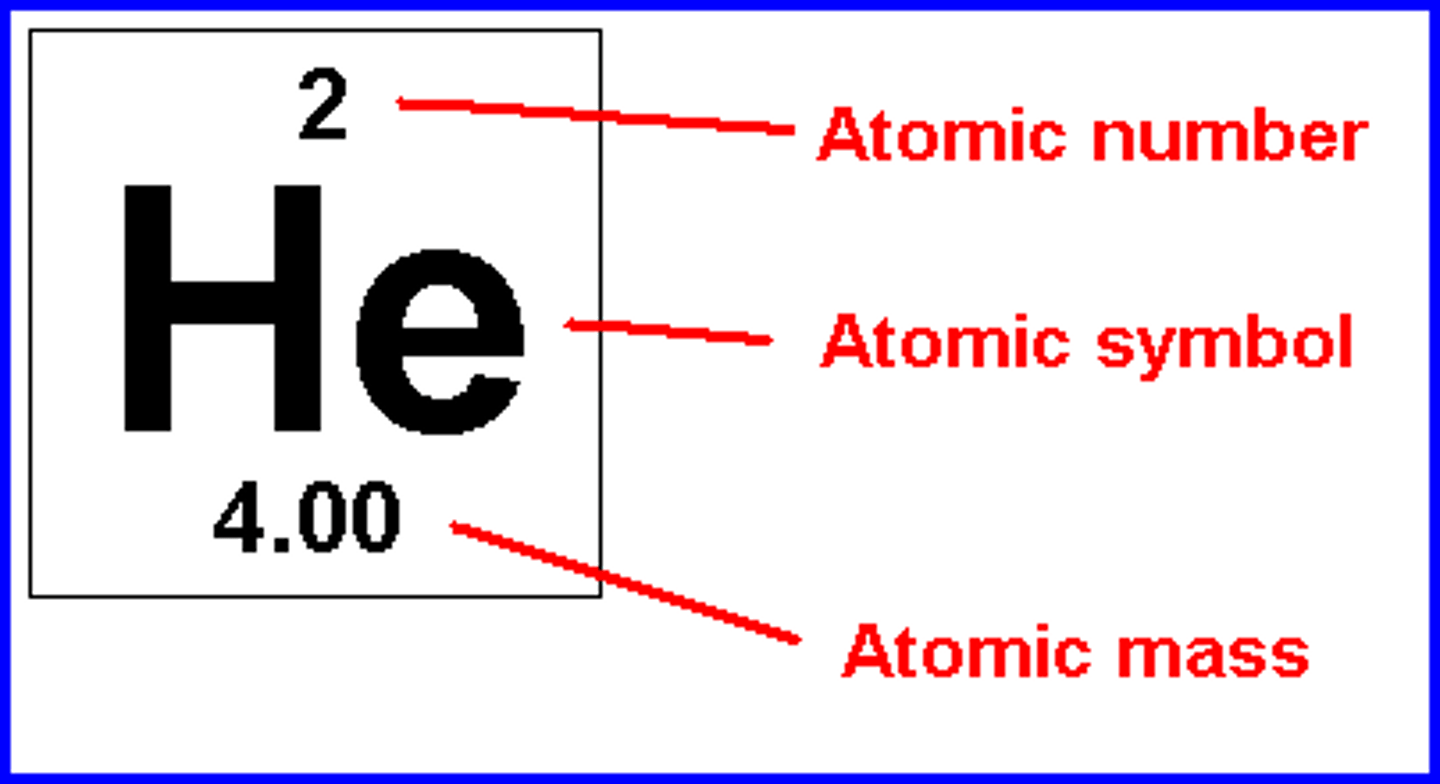
Atomic Number
The number of protons in the nucleus of an atom of an element

Mass Number
The total number of protons and neutrons in an atom's nucleus

Periodic table
A chart of all chemical elements currently known, organized by their properties.
Element
pure substance that consists entirely of one type of atom
atom
the smallest unit of an element that maintains the properties of that element
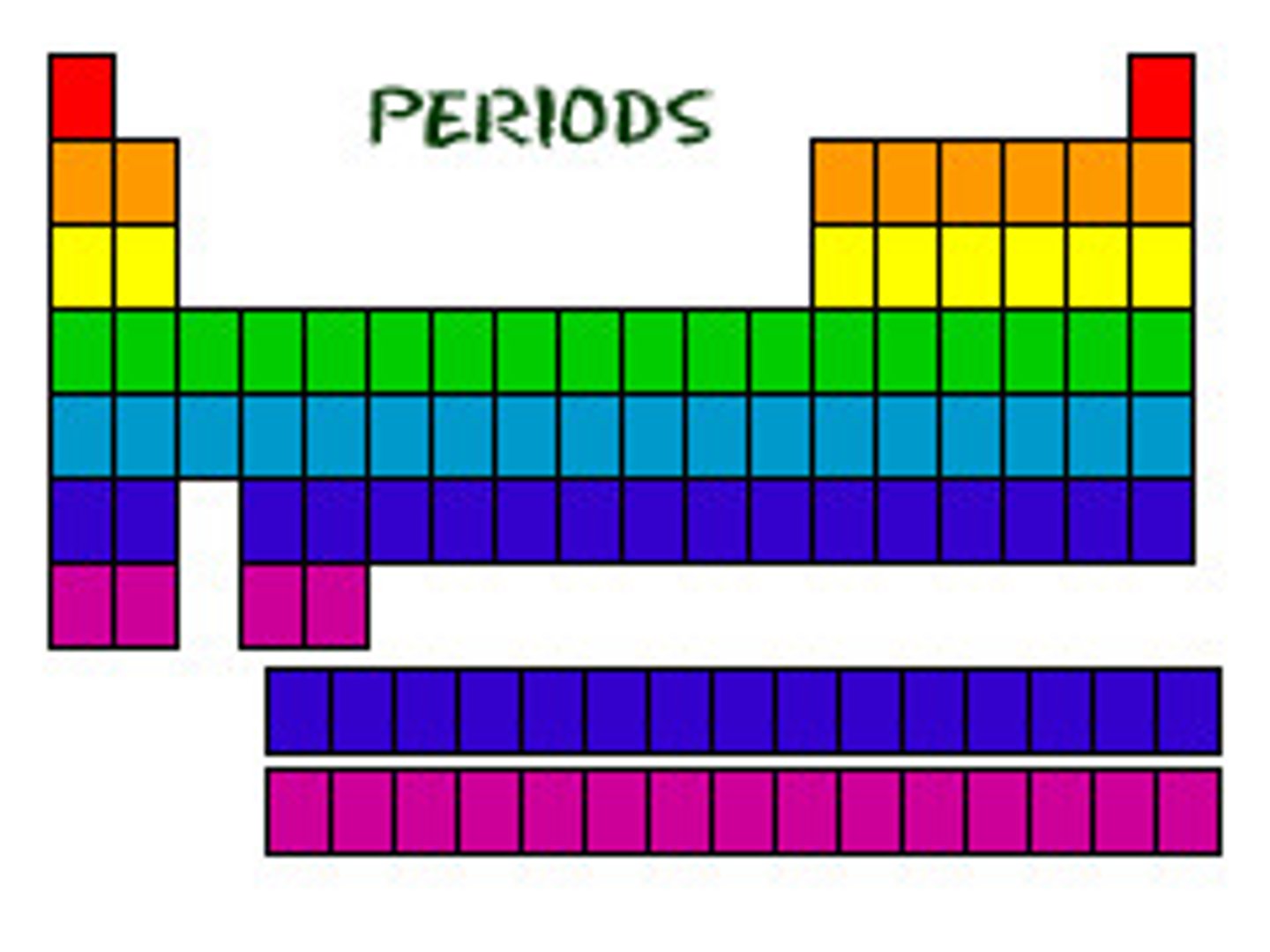
periods
horizontal rows on the periodic table
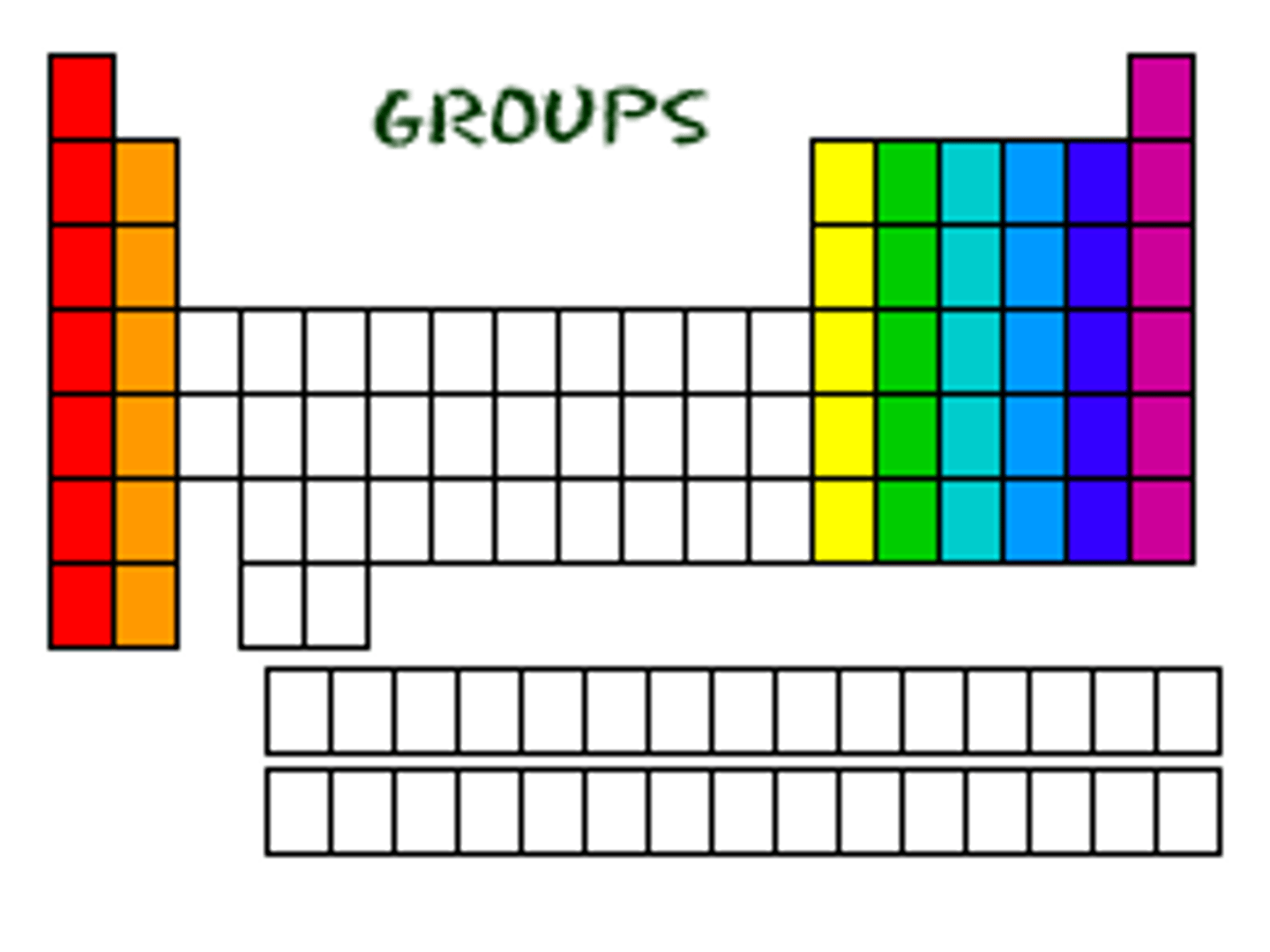
groups
vertical columns on the periodic table that have common properties
metal
shiny, malleable, ductile and good conductors
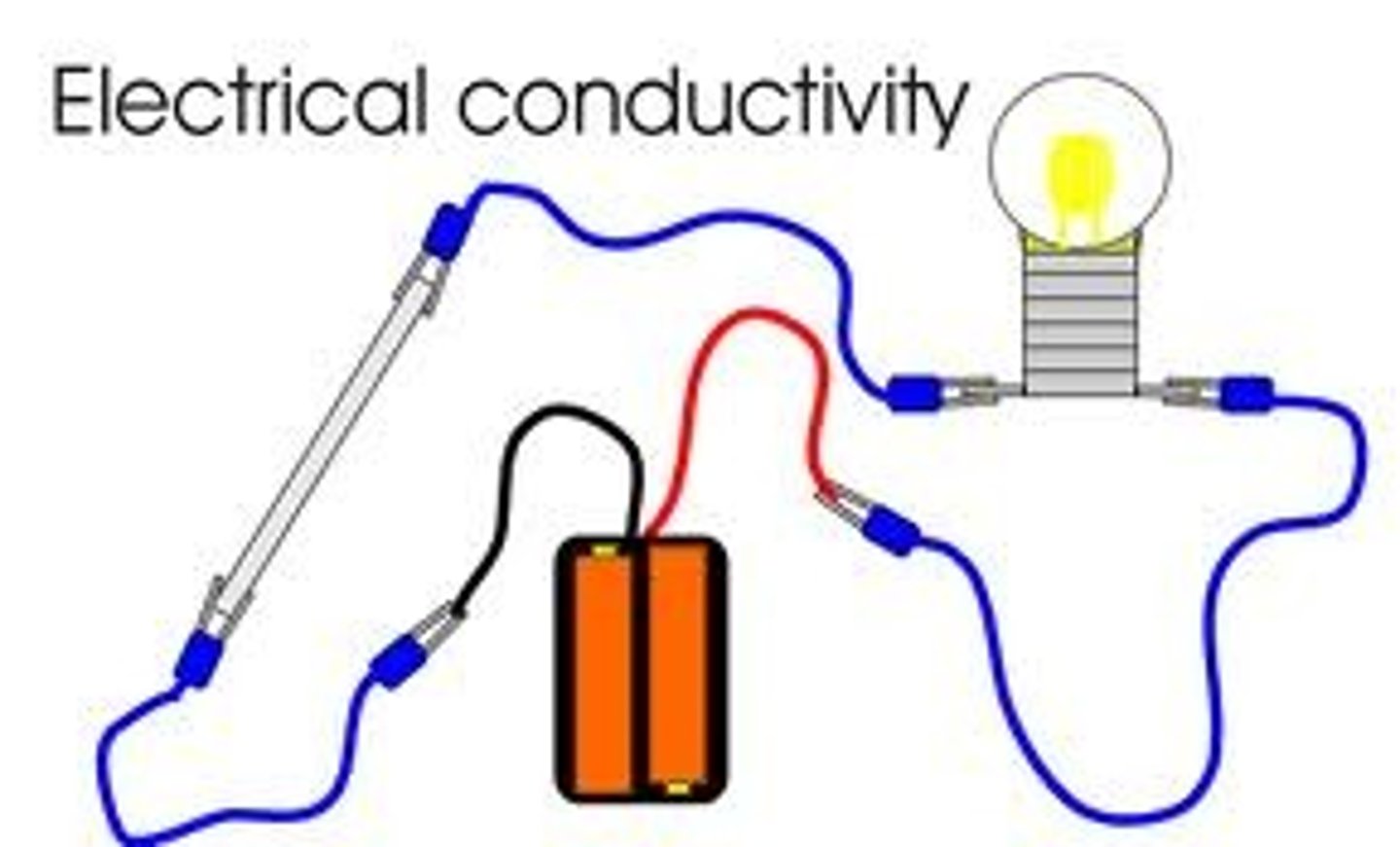
conductivity
the ability of an object to transfer heat or electricity to another object
reactivity
the ease and speed with which an element combines or reacts with other elements and compounds
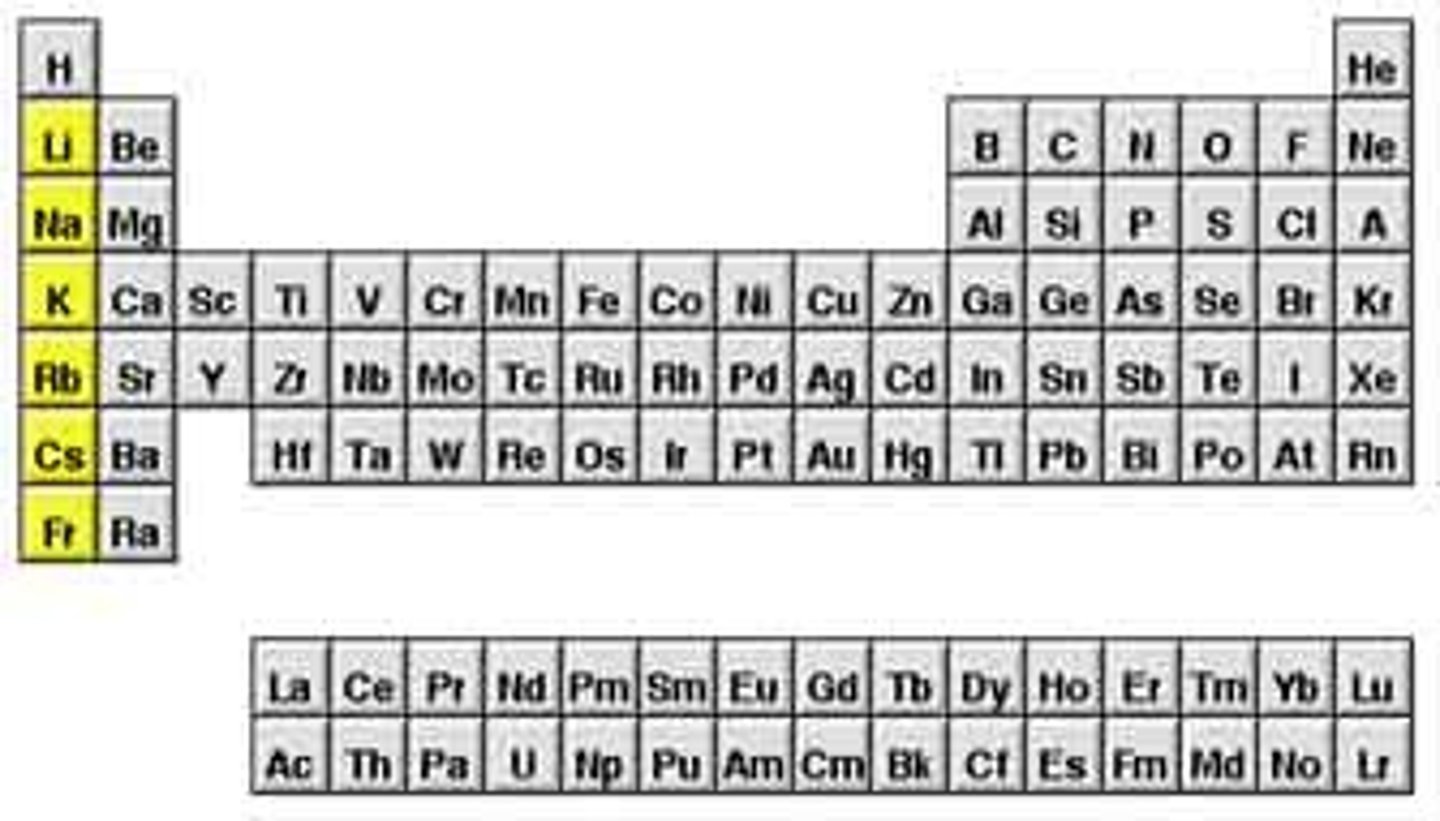
alkali metals
very reactive, not found alone in nature, react violently with water
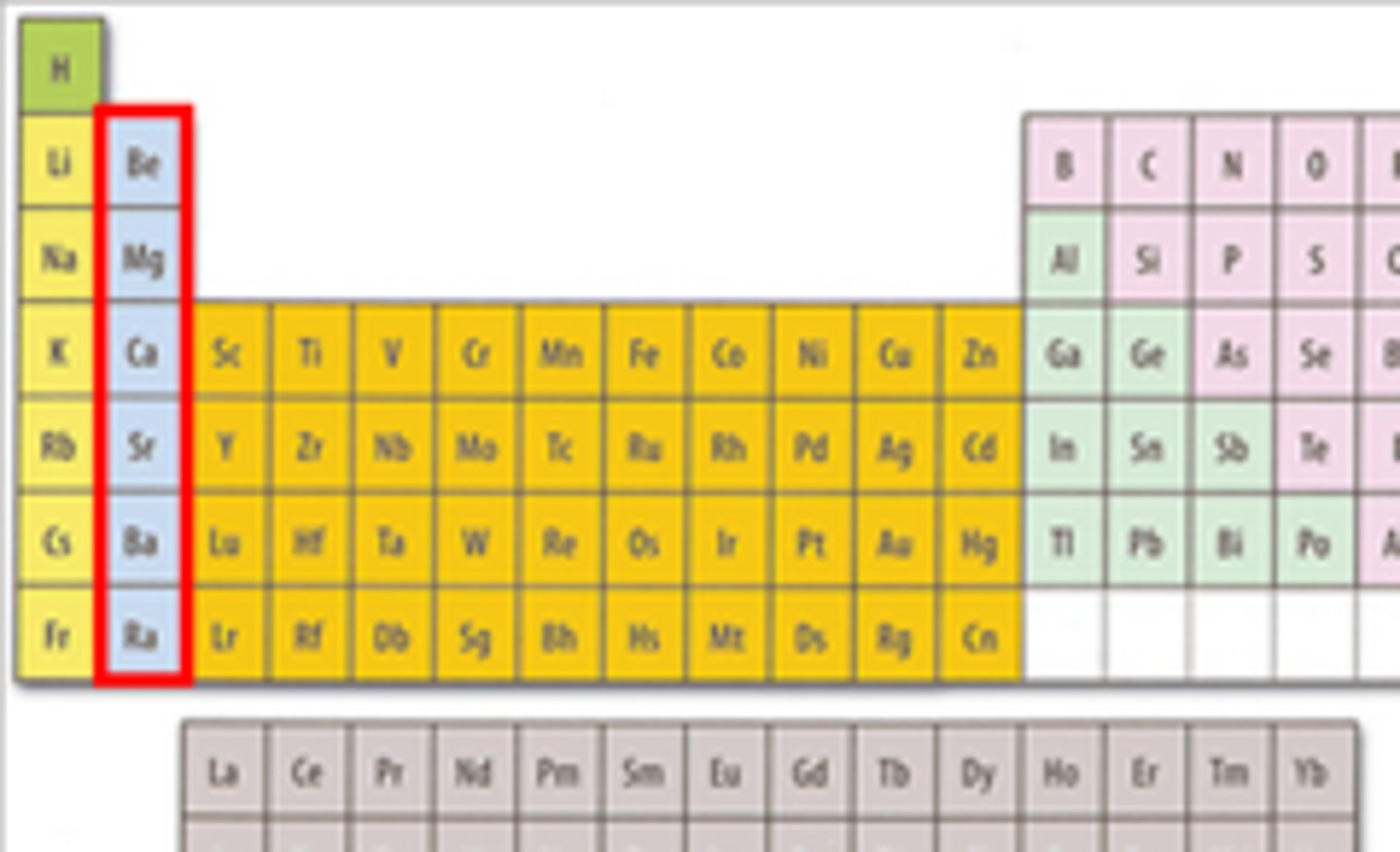
alkaline earth metals
hard, grey-white, good conductors of electricity, calcium and magnesium are examples
Transition metals
most are hard and shiny, less reactive, examples are iron, copper, nickel and gold
synthetic elements
not found naturally on earth, all elements higher than 92

Bohr
discovered that electrons follow an orbit/shell
Dalton
discovered that atoms of the same element have the same mass
Covalent bond
the sharing of electrons
Ionic bond
the transfer of electrons
Ion
forms when an atom loses or gains an electron
Isotope
the protons remain the same as the atomic number, but the neutrons do not
Noble gases
elements in group 18 that have complete outer shells
Halogen
group 17
Valence electrons
electrons that are located the farthest away from the nucleus- determine bonding
Radioactive isotopes
are unstable
Period number
is the amount of energy levels (shells) an atom has
atomic mass
weighted avg of all naturally occurring isotopes of that element; number on periodic table below chemical symbol