Biochemistry - Chapter 8 - Kinetics and Regulation
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Rate of a Reaction
The change in the amount of a reactant of product per unit time.
Rate Expression
Mathematical representation of rate of reaction.
When the velocity of a reaction is directly proportional to reactant concentration, the reaction is called a…
…first-order reaction, and the proportionality constant has the units s-1.
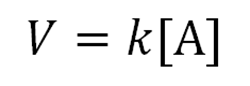
Integrated Rate Law Formula
Rate = k[A]n[B]m
The rate equations for the these reactions are…
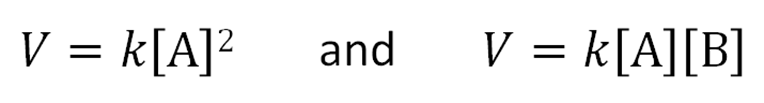
The proportionality constant for second-order reactions has the units M−1s−1.
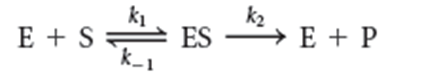
Under these conditions, the velocity is called…
…the initial velocity or Vo.
What is the Michaelis-Menten equation?
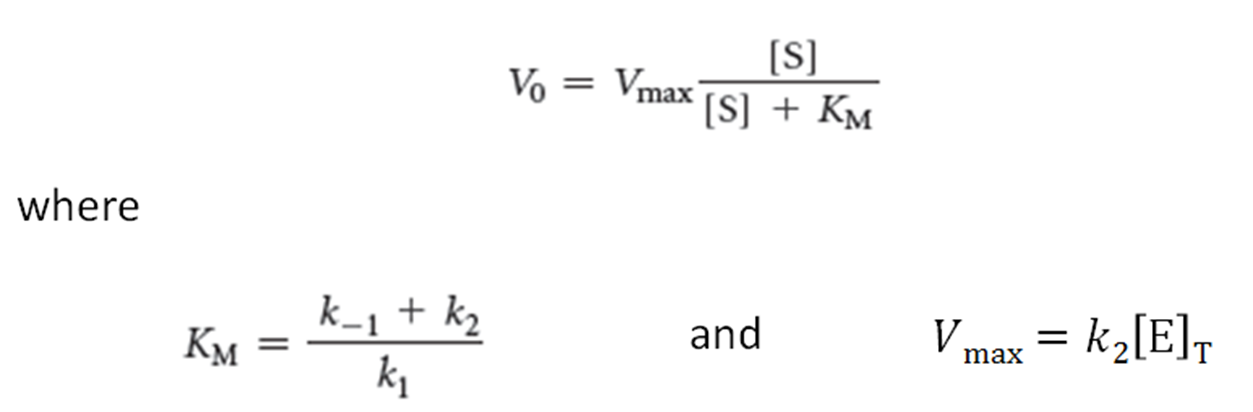
•When Vo = ½ Vmax, KM =[S]. Thus, KM is the substrate concentration that yields ½ Vmax.
Graph of Michaelis-Mentent Kinetics
•When Vo = ½ Vmax, KM =[S]. Thus, KM is the substrate concentration that yields ½ Vmax.
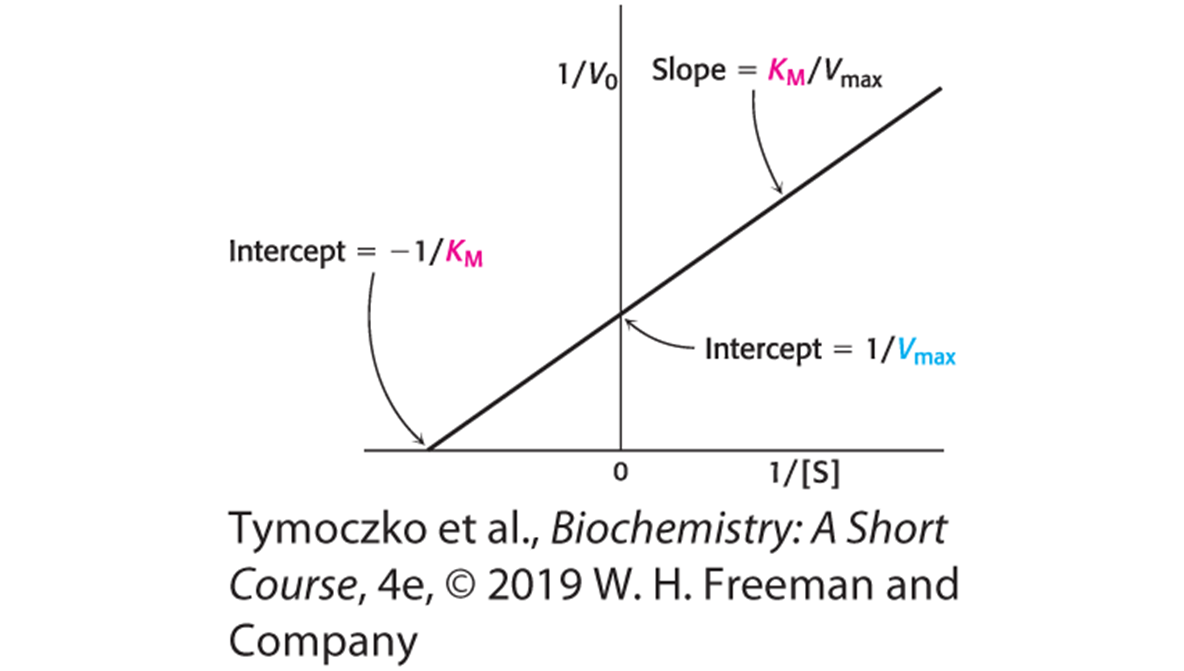
A Double-reciprocal, or Lineweaver–Burk, Plot
zyme concentration, [E]T, is known, then
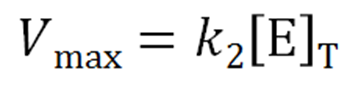
k2, also called kcat, is the turnover number of the enzyme, which is the number of substrate molecules converted into product per second.
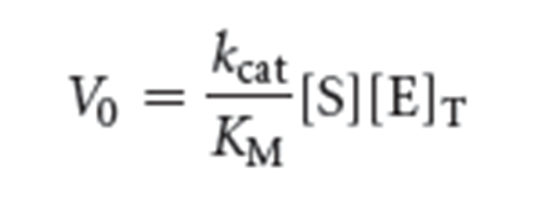
kcat/KM is a measure of catalytic efficiency because it takes into account both the rate of catalysis (kcat) and the nature of the enzyme substrate interaction (KM).
Multiple substrate reactions can be divided into two groups. What are these two groups?
Sequential reactions
Double-displacement reactions
Sequential reactions
Characterized by formation of a ternary complex consisting of the two substrates and the enzyme.; The first substrate (NADH) binds to the enzyme, followed by the second substrate (pyruvate) to form a ternary complex of two substrates and the enzyme. Catalysis then takes place, forming a ternary complex of two products and the enzyme. The products subsequently leave sequentially.
Double-displacement reactions
Characterized by the formation of a substituted enzyme intermediate; The first substrate (aspartate) binds, and the first catalytic step takes place, resulting in a substituted enzyme (E-NH3). The first product (oxaloacetate) then leaves.
The second substrate (a-ketoglutarate) binds to the substituted enzyme. The second catalytic step takes place and the NH3 is transferred to the substrate to form the final product glutamate, which departs the enzyme.
Allosteric enzymes control what?
The flux of biochemical reactions in metabolic pathways.
Because of their regulatory properties, allosteric enzymes allow for the generation…
of complex metabolic pathways.
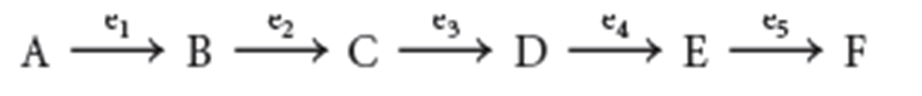
The conversion of A to B is the committed step because once this occurs,…
…B is committed to being converted into F.
Allosteric enzymes catalyze the what step of metabolic pathways. Michaelis–Menten enzymes facilitate what steps.
Allosteric enzymes: Committed
Michaelis–Menten enzymes: Remaining steps
The amount of F synthesized can be regulated by what?
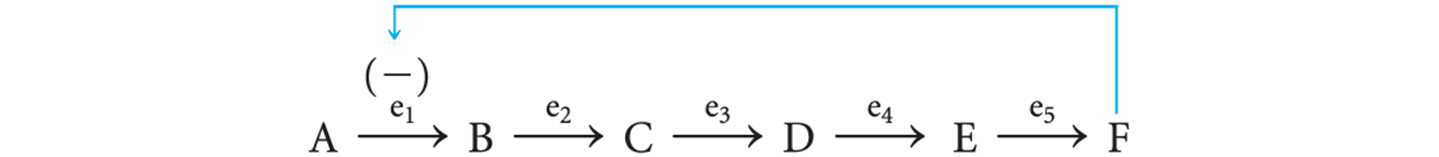
Feedback inhibition
The pathway product F inhibits enzyme e1 by binding to a regulatory site on the enzyme that is distinct from the active site.
Allosterically Regulated Enzymes Do Not Conform to Michaelis–Menten Kinetics
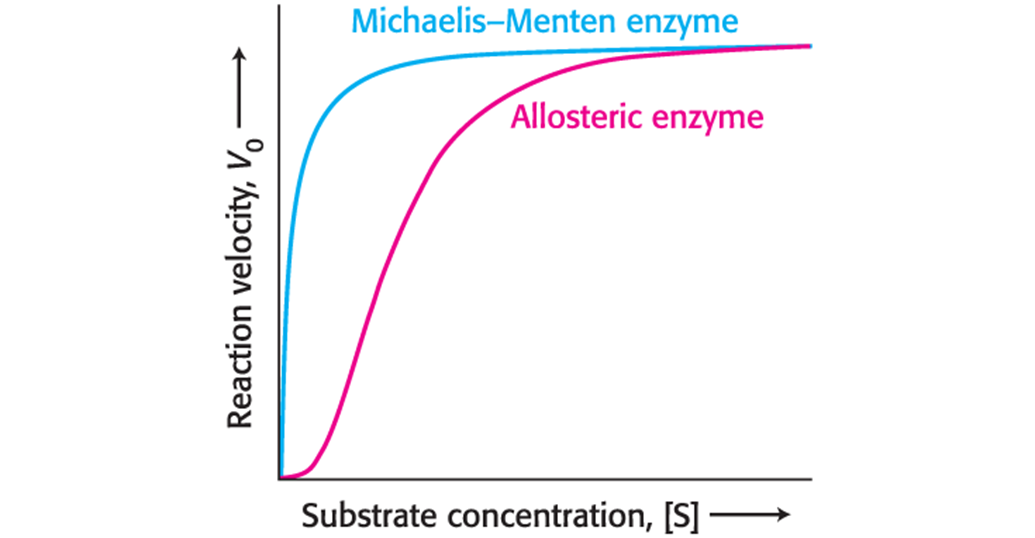
The reaction velocity of allosteric enzymes displays a sigmoidal relationship to substrate concentration.
All allosteric enzymes display…
…quaternary structure with multiple active sites and regulatory sites.
Features of the concerted model:
The enzyme exists in two different quaternary structures, designated T (tense) and R (relaxed).
T and R are in equilibrium, with T being the more stable state.
The R state is enzymatically more active than the T state.
All active sites must be in the same state.
The binding of substrate to one active site traps the other active sites in the R state and removes the substrate-bound enzyme from the T ⇌ R equilibrium.
This disruption of the T ⇌ R equilibrium by the binding of substrate favors the conversion of more enzymes to the R state.
Allosteric regulators disrupt the _______ when they bind the enzyme.
R ⇌ T equilibrium
Inhibitors stabilize the ___ state, whereas activators stabilize the ____.
T state
R state
Homotropic Effect
The disruption of the T ⇌ R equilibrium by substrates.
Heterotropic Effect
The disruption of the T ⇌ R equilibrium by regulators