Vaccination
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
what is natural acquired passive immunity?
- – Immunity acquired from antibodies passed in breast milk or through the placenta
First way of defence via antibodies
what is natural acquired active immunity?
- Immunity gained through illness and recovery
what is artificial acquired passive immunity?
- – Immunity gained through antibodies harvested from another person or animal
o Eg falling as a child and grazing your knee
what is artificial acquired active immunity?
- Immunity gained through a vaccine
Introduction of an antigen or an epitope
Describe what the immune response would be like to an ideal vaccine
- Upon first being introduced to an antigen, B-cells will produce many IgM antibodies via plasma cells (no T-cell assistance required as it is thymus independent)
- B-cells will mature into plasma and memory cells
- The first response is slow as there are no memory cells and clonal expansion takes time
- On the secondary exposure, there are many memory cells readily available, which differentiate into plasma cells to produce IgG antibodies, which is faster
- The ideal vaccine will produce a rapid secondary response
Describe what happens in humeral immunity
- B-cell encounters the antigen via the B-cell receptor on its surface
o If the antigen is complex enough, it is a thymus independent antigen as it is sufficient for the B-cell to differentiate to become a plasma cell and produce the antibodies itself
o In a vaccine, it would be ideal to have an antigen that is thymus independent so cell-mediated help is not required
- If the antigen is thymus-dependent, T-helper cells (TH2-cells) stimulates a B-cell to make antibodies
o The B-cell presents it’s antigen on the surface along with MHC class II molecule, which the TH2 cell recognises and binds to
o This is stabilised by a CD4 molecule
o The CD4 stimulates the TH2 to secrete mitogens that activate the B-cell to make and secrete antibodies
o Prior to a vaccination, there are not many of these specific TH2 cells to amplify this immune response
Describe what happens after phagocytic cells present the antigens on their surface?
- Phagocytic cells (neutrophils) will oxidise the bacterial cell and present the debris on its surface
o The bacterial cell would have been opsonised with C3b or C5b
o These antigen presenting neutrophils become a target for TH2 cells
o The antigens on the surface are stabilised on the surface via MHC class II, which allows the TH2 cells to recognise it
o The antigen presenting cell (APC) will release IL-1, which stimulates T-helper cells to divide, amplifying the immune response
o APC is stabilised with MHC class I, allowing TH1 cells to bind
o These then secrete mitogens, causing an upregulation of function of the APC (increasing the amount of phagocytosis)
How can the cell-mediated response be turned on?
o TH1 cell can stimulate an effector cell, which will mature into a cytotoxic T-cell
o TH1 cell secretes interferon alpha and beta, which causes the effector cells to differentiate into cytotoxic T-cells
o These recognise virally infected cells via the T-cell receptor (CD8)
o Cytotoxic T-cells recognise the antigen presented on the surface of a virally infected cell, which has been stabilised by a MHC class I molecule
- An effective vaccine will have a T-cell and B-cell response (humoral and cell-mediated adaptive immunity)
what is a live attenuated vaccine?
weakened strain of the whole pathogen
what are th advantages of a live attenuated vaccine?
§ Stimulates cell-mediated and humoral immunity
§ Long-lasting immunity
§ Transmission to contacts – can go from one person to another so immunity can be passed on
what are the disadvantages of a live attenuated vaccine?
§ Difficult to store and transport
§ Risk of infection to immunocompromised patients
§ Risk of reversion
o Eg chickenpox; German measles; measles; mumps; TB; typhoid fever; yellow fever
what is an inactivated vaccine?
whole pathogen killed or inactivated by heat, chemicals or radiation
what are the advantages of an inactivated vaccine?
§ Ease of storage and transport
No risk of severe active infection
what are the disadvantages of an inactivated vaccine?
§ Weaker immunity (humoral only) – only works with thymus independent antigens
· The structure of the antigens have been changed so much, it is difficult to mount a strong cell-mediated response
§ Higher doses and more boosters required
o Eg cholera; hep A; influenza; plague; rabies
what is a subunit vaccine?
- immunogenic antigens (using one part of the antigen or a particular epitope)
what are the advantages of a subunit vaccine?
lower risk of side effects
what are the disadvantages of a subunit vaccine?
§ Limited longevity
§ Multiple doses required
§ No protection against antigenic variation – one antibody to one epitope
§ Polyclonal would be more efficient
o Eg anthrax; hep B; influenza; meningitis; papillomavirus; pneumococcal pneumonia; whooping cough
what is a toxoid vaccine?
inactivated bacterial toxin
what are the advantages of a toxoid vaccine?
§ Humoral immunity to neutralise toxin
what are the disadvantages of toxoid vaccines?
§ Does not prevent infection
o Eg botulism; diptheria; pertussis; tetanus
what is a conjugate vaccines?
- capsule polysaccharide conjugated to protein (conjugate the antigen with something highly immunogenic)
what are the advantages of a conjugate vaccine?
§ T-dependent response to capsule
§ Better response in young children
what are the disadvantages of a conjugate vaccine?
§ Costly to produce
§ No protection against antigenic variation
§ May interfere with other vaccines
o Eg meningitis; Haemophilus influenzae; Streptococcus pneumoniae; Neisseria meningitides)
what is a DNA or mRNA subunit vaccine?
- synthesis of linear DNA that encodes the antigen
how do mRNA or DNA vaccines work?
- synthesis of linear DNA that encodes the antigen
o This is then used to synthesise mRNA, which is packaged into a formulation and injected
o This can be packaged with a reporter marker, which will target certain types of cells
o The mRNA can be injected into a phagocytic cell to display with MHC class I to stimulate a CD8 molecule, which stimulates cytotoxic T-cells
o Another method is using a bacterial cell to produce the mRNA which would then be formulated into a vaccine and injected
§ This would then have the proteins expressed on an APC with MHC class II
§ This would stimulate TH2
what are th advantages of mRNA or DNA vaccines?
§ Very quick to produce as the DNA sequences are easy to identify and the RNA sequences are easy to synthesise
Describe the composition of vaccines
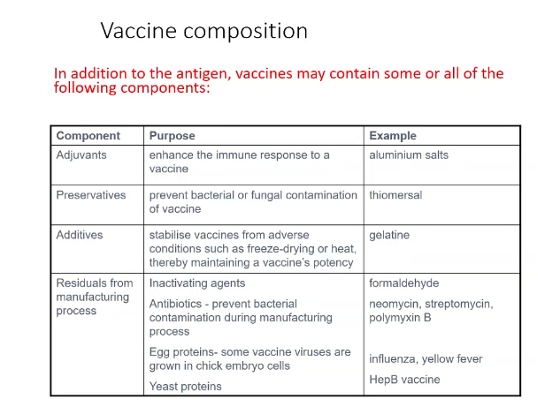
what is the effect of oil in water adjuvants in vaccines?
- can enhance the recruitment of immune cells to the injection site, promoting a stronger immune response
What is the aim of an immunisation programme and give examples
- To eradicate, eliminate or contain a disease – mass immunisation strategy
o Eradication – smallpox has been removed worldwide
o Elimination – polio has disappeared from WHO region but remains elsewhere
o Containment – Hib no longer constitutes a significant health problem