Physics Unit 7: Modern Physics
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What does quantum mean?
Discrete minimums for certain fundamental quantities like charge, momentum, angulra momentum, energy, etc
Waves vs Particles in Classical Theory
Waves explain reflection, refraction, interference, diffraction
Particles explain reflection, refraction, the photoelectric effect
Photons are NOT classical because Energy is proportional to the frequency, NOT the amplitude
If a wave has lower energy but a higher power, then the # of photons must be higher.
De Broglie Formula
λ = h/p = h/mv
or alternatively, p = sqrt(2Em) => h/sqrt(2Em) = λ
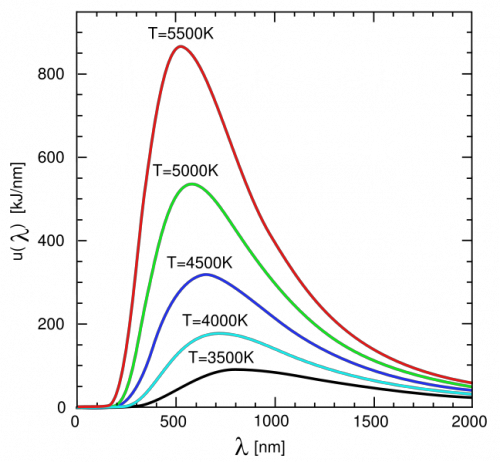
Blackbody Radiation
Blackbody: something that absorbs all incident radiation and emits a spectrum of continuous radiation that is INDEPENDENT of the initial amount or type of radiation. An ideal blackbody stays at constant temperature, absorbing none and emitting all so 0 net flux in or out.
P = AσT4 , λmax = b/T (in KELVIN)
Therefore, the hotter a blackbody, the lower the peak wavelength, the higher the frequency (blue shift)
Blackbody Radiation Graph
Wavelength vs intensity (flux or power/area)
Continuous spectrum with peak wavelength (the color you see). As you move right, the wavelength increases and temperature DECREASES.
Increasing radiation corresponds to a more left peak that is higher and more narrow.
A stationary observer sees light. An observer on a train moving at 1 million m/s see light. How does the speed of light differ?
the speed of light for both observers (relative velocity) is c. This is due to quantum mechanics and relativity, the two observers are experiencing time at different rates.
Electron orbiting a nucleus
Fe = Fc => kq1q2/r2 = mv2/r
(note: use only the proton number in Coulombs, NOT atom mass number for q)
v ~ 1/r ~ 1/PE
Thus, the radius of orbit depends on the velocity of the electron. Electron can be treated also as a wave and for the electron to exist in the discrete energy levels it does without falling into the nucleus, it must have energy levels that are whole numbers of the electron’s De Broglie wavelength
Absorption/Emission Spectra
Electrons transition from ground/excited state when emitting or absorbing light at discrete energy levels. release photon because of the accelerating charge. Questions may ask about the total number of transition states or energy of photon (highest wavelength = lowest energy transition differences, etc)
What happens if the energy of an incident photon is greater than any difference between energy states?
The atom is ionized with excess energy becoming the KE of the ejected electron.
Photoelectric Effect
Shows wave-particle duality
Einstein’s encapsulated quartz tube experiment showed that
Kmax = nhf - Φ (work function or ionization energy of electron).
Electrons ejected depends on the frequency/energy (represented by the voltage of a battery). It does NOT depend on intensity (# of photons or current through circuit)
The threshold frequency is the frequency that allows and electron to be ejected with 0 kinetic energy
Brightness is proportional to # of photons
Flux is # photons/time
Intensity is I = P/A or nhf/(t*A)
Compton Effect
p = h/λ. When a photon collides nonelastically with an electron/fundamental particle, it gives off energy and momentum.
-the electron experiences a change in momentum and speed, and accelerates which produces and EM wave (another photon)
-The wave propagated after the collision has less energy (some was transferred to electron) so less frequency and greater wavelength
-the momentum of the photon does NOT change because photons have no mass and ALWAYS travel at the speed of light
Heisenberg Uncertainty Principle
∆x∆p = h/4π
The more certain your are about one quantity, the less certain you can be about the other
Quantum Entanglement
Think: Schrodinger’s cat
Two or more objective even if far away can be treated as a single system, and knowing something about one object in the system tells us something about the other object
Schrodinger’s Equations
Just know that the probability of finding an electron is the square of the wave function (Ψ²)