Farahat Chapter 19 MCQ
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
True or False? Transition metals show great similarities both within a given period and within a given vertical group.
True
What is the maximum oxidation state of chromium?
a. +4
b. +6
c. +3
d. +5
e. none of these
b. +6
What transition metal is used in magnets, catalysts, and drill bits?
a. platinum
b. nickel
c. copper
d. cobalt
e. titanium
d. cobalt
The phenomenon called the __________ contraction is responsible for the great similarity in atomic size and chemistry between 4d and 5d elements.
a. transition
b. coordination
c. none of these
d. isomeric
e. lanthanide
e. lanthanide
5. Which metal ion has a d4 electron configuration?
a. Ti2+
b. Mn2+
c. Ni2+
d. Fe3+
e. Mn3+
e. Mn3+
Which of the following is a d2 ion?
a. Mn3+
b. Ti+
c. Fe3+
d. Cr2+
e. Ti2+
e. Ti2+
What is the electron configuration of the Co(II) ion?
a. [Ar]3d6
b. [Ar]3d7
c. [Ar]4s23d5
d. [Ar]4s24d2
e. [Ar]4s23d7
b. [Ar]3d7
Which of the following is a d7 ion?
a. Co2+
b. Zn2+
c. Fe2+
d. Cr3+
e. Cu+
a. Co2+
A compound of which transition metal is responsible for the white color of most paper?
a. chromium
b. titanium
c. zinc
d. nickel
e. copper
b. titanium
Which transition metal can exist in all oxidation states from +2 to +7?
a. iron
b. vanadium
c. copper
d. manganese
e. chromium
d. manganese
The corrosion of which transition metal results in a characteristic green patina?
a. lead
b. copper
c. chromium
d. silver
e. iron
b. copper
Which of the following statements is true about coordination complexes?
a. When the ligands approach a transition metal ion in an octahedral field, the dxz, dyz, and dxy atomic orbitals are affected the least by the ligands.
b. The metal is a Lewis base and the ligands are Lewis acids.
c. Only complexes with coordination number 6 are found in nature.
d. None of these is true.
e. All of these are true.
a. When the ligands approach a transition metal ion in an octahedral field, the dxz, dyz, and dxy atomic orbitals are affected the least by the ligands.
Which of the following coordination compounds will form a precipitate when treated with an aqueous
solution of AgNO3?
a. Na3[CrCl6]
b. [Cr(NH3)3Cl3]
c. Na3[Cr(CN)6]
d. [Cr(NH3)6]Cl3
e. all of these
d. [Cr(NH3)6]Cl3
How many d electrons are present on the metal ion in the complex ion PtCl6
2–
?
a. 3
b. 8
c. 2
d. 4
e. 6
e. 6
Which of the metal ions in the following complex ions has a d5 electron configuration?
a. [FeCl6]4–
b. [Cr(CN)6]3–
c. [Ti(H2O)6]2+
d. [Mo(NH3)6]3+
e. [Fe(CN)6]3-
e. [Fe(CN)6]3-
In which of the following complexes does the transition metal have a d8 configuration?
a. [Zn(NH3)4]2+
b. [NiBr4]2–
c. [Mn(CN)6]3-
d. [Ni(CO)4]
e. [Ti(H2O)6]2+
b. [NiBr4]2–
_____ isomers and _______ isomers are classes of structural isomers.
a. Geometric, optical
b. Geometric, linkage
c. Linkage, geometric
d. Coordination, linkage
e. Coordination, geometric
d. Coordination, linkage
Which of the following are structural isomers?
I. coordination isomers
II. linkage isomers
III. geometric isomers
IV. optical isomers
a. II, IV
b. II, III
c. I, II
d. I, III, IV
e. I, III
c. I, II
Which of the following complexes shows geometric isomerism?
a. K[Co(H2O)2Cl4]
b. [Co(H2O)5Cl]Cl2
c. [Co(H2O)5Cl]SO4
d. Na3[CoCl6]
e. [Co(H2O)6]Cl3
a. K[Co(H2O)2Cl4]
Which model(s) account(s) for the magnetism and color of coordination compounds?
I. the localized electron model
II. the crystal field model
a. both I and II
b. neither I nor II
c. II only
d. I only
c. II only
The complex FeL62+, where L is a neutral ligand, is known to be diamagnetic. How many d electrons are there in this complex ion?
a. 7
b. 8
c. 6
d. 4
e. 5
c. 6
Fluoride ion ranks low in the spectrochemical series and produces a weak crystal field in complex ions. Based on this information, predict the number of unpaired electrons in [CoF6]3–.
a. 0
b. 1
c. 2
d. 3
e. 4
e. 4
The complex ion [NiCl4]2– is tetrahedral. How many unpaired electrons are there in the complex?
a. 4
b. 1
c. 2
d. 3
e. 0
c. 2
A metal ion in a high-spin octahedral complex has two more unpaired electrons than the same ion does in a
low-spin octahedral complex. Which of the following could the metal ion be?
a. Cr3+
b. Mn2+
c. Co2+
d. Ti2+
e. Cu2+
c. Co2+
For which of the following metal ions would there be no distinction between low spin and high spin in octahedral complexes?
a. Cr2+
b. Mn2+
c. Ni3+
d. V2+
e. Co3+
d. V2+
How many unpaired electrons are there in a complex ion having a d5 electron configuration and an octahedral geometry in the weak-field case?
a. 2
b. 1
c. 3
d. 5
e. 4
d. 5
The spectrochemical series is
I–< Br–< Cl–< F–< OH–< H2O < NH3 < en < NO2–< CN–
Which of the following complexes will absorb visible radiation of the highest energy (shortest wavelength)?
a. [Co(OH)6]3–
b. [Co(NH3)6]3+
c. [Co(I)6]3–
d. [Co(H2O)6]3+
e. [Co(en)3]3+
e. [Co(en)3]3+
Which of the following statements is true of the crystal field model?
a. The electrons are assumed to be localized.
b. The metal ion–ligand bonds are considered completely ionic.
c. The ligands are treated as negative point charges.
d. The interaction between metal ion and ligand is treated as a Lewis acid–base interaction.
e. None of these statements is true.
c. The ligands are treated as negative point charges.
The color of a transition metal complex results from
a. nuclear magnetic resonance.
b. transition of an electron between d orbitals.
c. bending vibrations.
d. stretching vibrations.
e. transition of an electron between an s orbital and a p orbital.
b. transition of an electron between d orbitals.
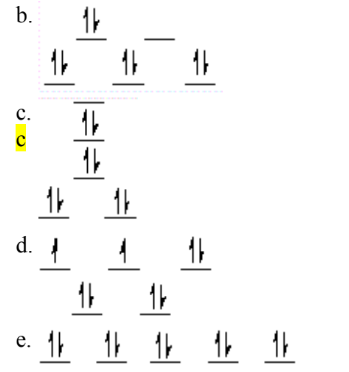
A complex ion is a square planar complex. It has a d8 electron configuration. What is the most reasonable d orbital scheme for this complex?
C
Which of the following complexes would be diamagnetic (all electrons paired)?
a. [Fe(CN)6]4–
b. [Mn(CN)6]4–
c. [V(CN)6]3–
d. [Cr(CN)6]3–
a. [Fe(CN)6]4–
How many unpaired electrons are there in the complex ion [Co(NO3)6]4–? For this ion, the nitrate ligands produce a very strong crystal field.
a. 4
b. 5
c. 3
d. 2
e. 1
e. 1
How many unpaired electrons are found in NiBr42– (tetrahedral)?
a. 0
b. 4
c. 1
d. 5
e. 2
e. 2
Which of the following is paramagnetic?
a. [Co(NH3)6]3+ (strong field)
b. [Cu(en)3]+
c. [Mn(CN)6]4–(strong field)
d. [Fe(CN)6]4–(strong field)
e. [Zn(H2O)6]2+
c. [Mn(CN)6]4– (strong field)
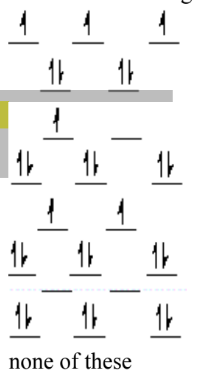
Which of the following crystal field diagrams is correct for Co(CN)64–, where CN– is a strong-field ligand?
B
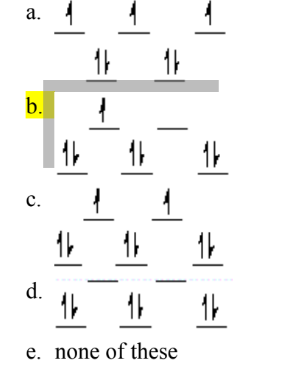
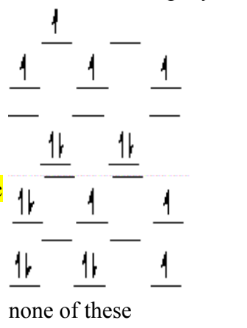
Which of the following crystal field diagrams is correct for Mn(CN)63–, where CN– is a strong-field ligand?
C
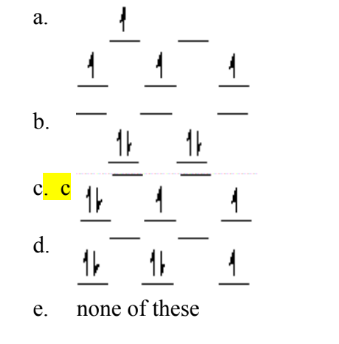
The complex ion Fe(CN)64– (no unpaired electrons) is classified as
a. There is no way to tell.
b. strong field.
c. weak field.
b. strong field.
The complex ion Co(NH3)62+ (three unpaired electrons) is classified as
a. There is no way to tell.
b. strong field.
c. weak field.
c. weak field.
Which of the following statements is true about the octahedral complexes of Ni2+?
a. Both strong- and weak-field complexes are paramagnetic.
b. The strong-field complex is paramagnetic and the weak-field complex is diamagnetic.
c. Both strong- and weak-field complexes are diamagnetic.
d. The strong-field complex is diamagnetic and the weak-field complex is paramagnetic.
a. Both strong- and weak-field complexes are paramagnetic.
Oxygen is stored in mammalian tissue in which type of molecule?
a. chlorophyll
b. cytochrome
c. hemoglobin
d. prophyrin
e. myoglobin
e. myoglobin
Which of the following transition metals is a component of vitamin B12?
a. chromium
b. zinc
c. copper
d. manganese
e. cobalt
e. cobalt