Chemistry - Acid base reactions
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Acid
a substance that donates a H+ proton
Base
a substance that accepts a H+ proton
Acidic proton
a hydrogen atom within a molecule that is readily released as a positively charged hydrogen ion (H+)
Monoprotic acid
acids that donate one proton eg. HSO4
Diprotic acid
acids that can donate 2 protons eg. H2SO4
Triprotic
H3PO4 - acids that can donate 3 protons
Amphiprotic
can act as an acid or base eg. HSO4 (-) can become SO4 (2-) or H2SO4
Conjugate acid-base pair
acid → conjugate base ( donates a proton )
base → conjugate acid ( accepts a proton )
acid-base reaction involves an exchange of protons from an acid to a base
Brønsted-Lowry theory
The describes acid-base properties in terms of proton (H+) transfer.
A Brønsted-Lowry acid is a proton donor, and a base is a proton acceptor.
Molecular dipole
the separation of electrical charge within a molecule, creating a partially positive end and a partially negative end
hydronium ion
H3O+ (when H2O gains a proton)
Neutralisation reaction
a chemical reaction in which an acid and a base react quantitatively to produce a salt and water
pH scale
a measure of how acidic or basic (alkaline) a substance is
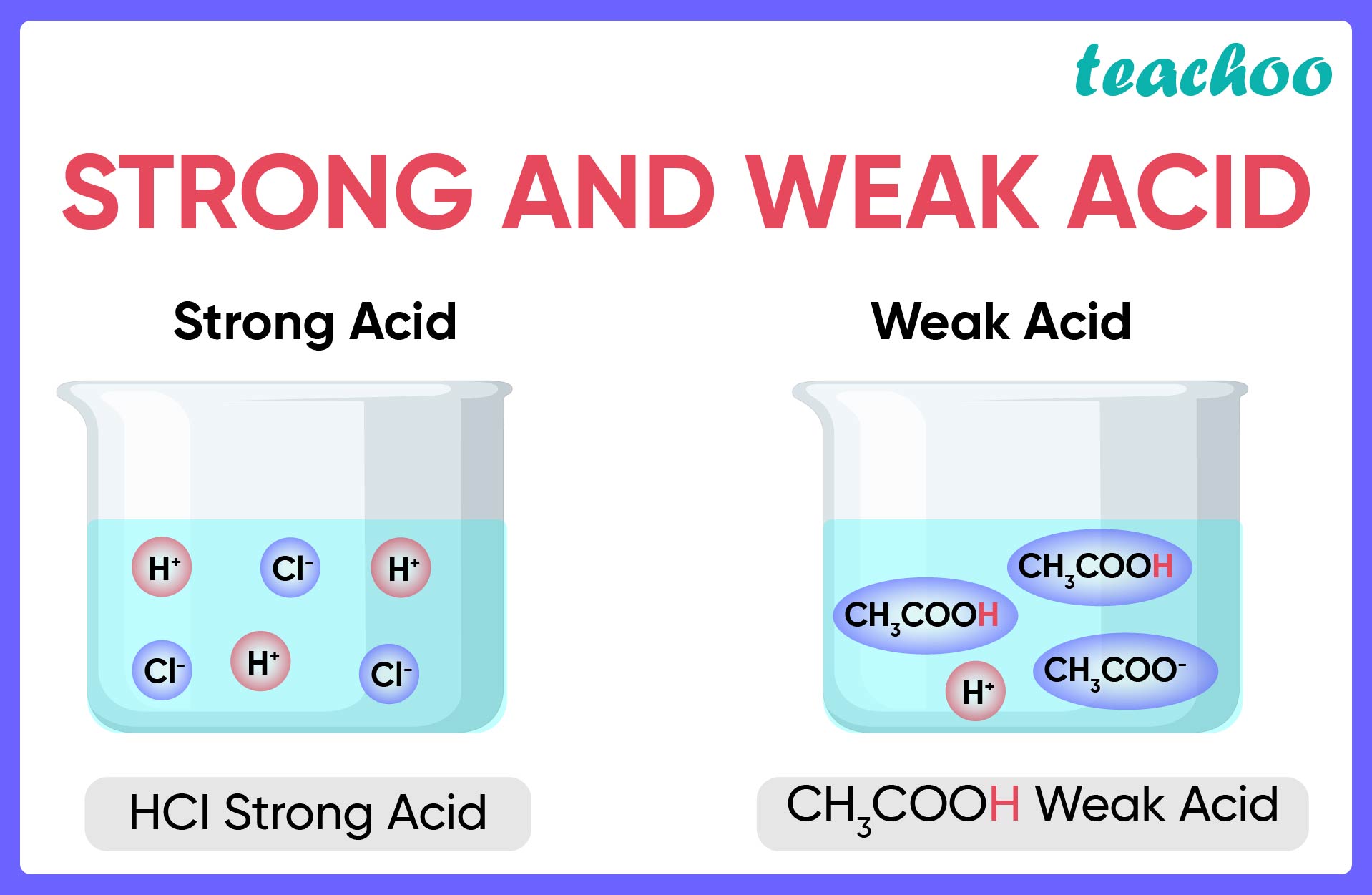
strong acid
readily donates H+, meaning there is a high percentage ionisation, 99% of the acid molecules break apart into ions
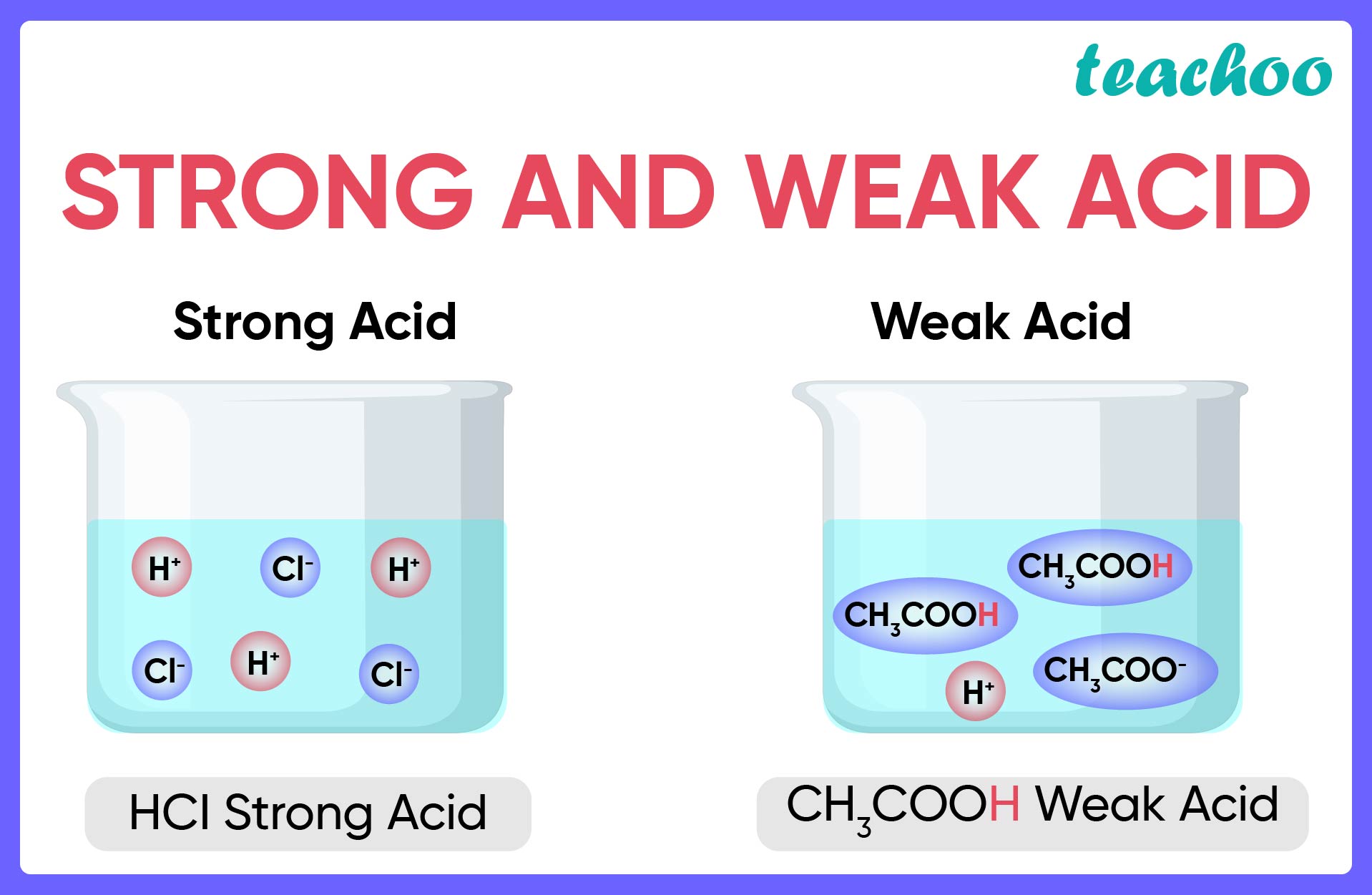
weak acid
only 1% of the molecule ionises, do not readily donate H+, low percentage ionisation
properties of acids
h+ donor
pH < 7
sour taste
turn litmus red
corrosive (destroys living tissue and metal)
neutralised by bases
properties of bases
H+ acceptor
pH > 7
turns litmus blue
bitter taste, feels slippery
caustic (destroys living tissue)
alkali = soluble in water
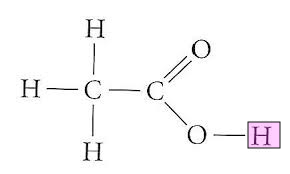
ETHANOIC ACID!!!!!
CH3COOH CAN ONLY LOOSE ONE PROTON/HYDROGEN
the other 3 are covalently bonded to the carbon so they can’t readily be lost
the proton is acidic because the oxygen is highly electronegative and attracts
the bonding electrons therefore weakening the bong to the proton allowing it to be donated
double arrow example
HSO4- is described as a weak acid because its only partially ionised as its able to loose one more to become SO4 to become fully ionised or H2SO4 where its fully ionised
therefore a double (reversible) arrow is used to indicate an incomplete reaction
calculating concentration
solute amount/solvent amount
strength vs concentration
strength - ability to ionise
concentration - how much of a substance is present within a solution.
dilute
not many particles
It means there's a large amount of solvent (like water) compared to the amount of solute (like salt or sugar) dissolved in it
concentrated
a lot of particles
A concentrated solution is one where a large amount of solute is dissolved in a relatively small amount of solvent,
solute
the substance being dissolved
solvent
the substance the solute is dissolved in
chemicals equation for strong acid/base dissociating in water example
HClO4 (aq) + H2O (l) — 99% ionisation → H3O+(aq) + ClO4 (aq)
write on top of the arrow 99% ionisation
chemicals equation for weak acid/base dissociating in water example
NH3 (aq) + H2O (l) — 1% ionisation → NH4+(aq) + OH- (aq)
write on top of the arrow 1% ionisation or use double arrow ( ⇌ )
Reactions of acids
Acid + Metal → Salt + H2 (g)
Acid + Oxide → Salt + H2O (l)
Acid + Carbonate → Salt + H2O (l) + CO2 (g)
Use solubility table when looking for the reactants states to know if its (aq) or (s) example
Ca (s) + HCl (aq) → CaCl2 (aq) + H2 (g)
aqueous because on the solubility table chloride is soluble with everything except some exceptions, however calcium is not an exception
Ag2O(aq) +HCl (aq) → AgCl (s) +H2O (g)
solid because on the solubility table chloride is insoluble with silver
Why does ca not have a charge in this equation?
Ca (s) + HCl (aq)→ CaCl2 (aq) + H2 (g)
because it is solid meaning there is no ions present
self ionisation of water
H2O + H2O → H3O+ + OH -
forms a hydronium ion (H3O) and hydroxide (OH-)
Ionisation constant for water
At 25°C: Kw=[H3O+] x [OH-] = 10-14 M2
Neutral solution the concentration of the hydronium ion and hydroxide ions are equal
acid added to water
when an acid is added the concentration of hydronium increases, whereas the concentration of hydroxide decreases
when a base is added to water
hydroxide increases and hydronium decrease
calculate concentration of acid
H3O+ (or H+) = 10-14/OH-
calculate concentration of base
OH- = 10-14/H3O+
changing calc from decimal to 1.69×10-6 M
ENG button
Spector ions
ions that don’t participate in the acid-base reaction
spectator ions
are ALWAYS AQUEOUS
aqueous to begin with and aqueous on the reactant side
eg. Mg (s) + H2SO4 (aq) → MgSO4 (aq) + H2 (g)
SO42- is the spectator ion
what does pH stand for?
potential hydrogen
strong acid, neutral, strong base
strong acid, = 1 or 10-1
neutral, = 7 or 10-7
strong base, = 14 or 10 -14
calculate pH
pH = - log10 [H3O+]
example
pH = - log10 (5×10-12)
pH = 11.3
or
pH = - log10 (0.0001)
pH = 4
calculate H3O from pH
[H3O+] = 10-pH
calculate the [H3O] of a solution with a pH=5.2
H+=10-5.2
H+ = 6.31 × 10-6 M
REMEMBER TO PUT WHAT AFTER H3O or OH-
M
calculate the pH of a solution of 0.001M magnesium hydroxide
Mg(OH)2 = 0.001M
OH- = 0.002M as in one mole of mg there are 2 OH- so 0.001 × 2
[H3O+]=10-14/0.002M
H3O+ = 5 × 10-12 M
pH = - log10(5×10-12)
pH=11.3
intermolecular forces
between different molecules
dispersion forces, dipole-dipole, hydrogen bonds
weakest to strongest bond ————>
dispersion forces
between all molecules
dipole-dipole
only when a polar bond is present ( BETWEEN 2 POLAR BONDS)
hydrogen bonding
only between H-NOF
nitrogen, oxygen, fluorine
intramolecular bonds
within the molecules
metallic - metal ions, free moving electrons sea of delocalised electrons
covalent - sharing of electrons
ionic - give and take of electrons
polar bonds
asymmetrical
difference in electronegativity
lone pairs
electron pairs that don’t partake in the bonding
aqueous
the ability to dissolve in water
potable
drinkable (only 0.3% is drinkable on earth)
waters bonding
water is a polar molecular that can from hydrogen bonds
has 2 lone pairs
bonds between the atoms are covalent bonds which only breaks when. chemical reaction takes place and are strong whereas hydrogen bonds are weak
hydrogen carries a partially positive charge
oxygen carries a partially negative charge
water- boiling point/melting point
high boiling/melting point due to the presence of hydrogen bonds
means it needs more heat energy to break the intermolecular bonds
main force needed to overcome to boil water - hydrogen bonds
max number of hydrogen bonds it can form
4
why does liquid water expand on freezing
it forms a regular pattern as the temp decrease as the kinetic engird decreases
why doesn’t water form 4 hydrogen bonds in a liquid state
due to the high kinetic energy due to movement atoms are moving around to quickly to form any type of regular structures, preventing them from having 4 hydrogen bonds as hydrogen bonds are forming and reforming
gas - water properties
high kinetic energy
no hydrogen bonds between water molecules
can not have a density
liquid - water properties
lower kinetic energy
hydrogen bonds from and reform
higher density
solid - water properties
hexagonal patterns trap more air- more space inside
less density
ice will float as it has less density then water due to the air trapped in the hexagonal patterns
specific heat capacity
amount of energy required to raise the temperature of 1g of the substance by 1 degree celsius
waters specific heat capacity
4.18 Joules per gram per degree celcuis
4.18 J.g-1.°C-1
4.18 joules is the required mount of energy to raise the temp of 1g of water by 1°C
properties of waters heat capacity
water absorbs large amounts of heat energy before it gets hots and slowly releases heat energy when it cools
it moderates earths temp and body temp
calculating heat energy
heat energy = specific heat capacity x mass (in grams ) x temperature change (°C)
q=m c △T
KJ → J
J → KJ
KJ → J x1000
J → KJ /1000
Kg → g
g → kg
Kg → g x1000
g → kg /1000
latent heat
energy absorbed by a fixed amount of substance as it changes state
latent heat of fusion
energy needed to convert water from a solid to a liquid ( at 0°C)
6.0095 KJ
latent heat of vaporisation
energy needed to convert water from a liquid to a gas ( at 100°C)
40.657 KJ
why is an ice pack used ons ore muscles
. latent heat of fusion helps to decrease the soreness/inflammation by absorbing the heat energy from the muscle to cool it down.