Year 10 GCSE Chemistry - Particles and Atomic Structure
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Element
Simplest type of substance
Where all elements are found
Periodic table
Atom
Smallest part of an element / that make up a substance that still has its chemical properties
Molecule
Particle made from multiple atoms joined together by chemical bonds
Bond
Attractive force holding atoms together in a molecule
Atomic radius
Distance from centre of an atom to outside
Bond length
Distance between centres of two joined atoms
Subatomic particles
Very small particles found inside an atom
Proton
Subatomic particle with relative mass 1 and charge + 1
Neutron
Subatomic particle with relative mass 1 and charge 0
Electron
Subatomic particle with relative mass 1 / 2000 and charge - 1
Nucleus
Centre of an atom containing protons and neutrons
Shells
Rings around the nucleus of an atom in which electrons are found
Why when you add water to orange squash, the orange colour spreads out
The two substance are made from tiny particles that can mix together
Matter
What everything is made from, including particles
What particles refer to
Atoms, ions and molecules
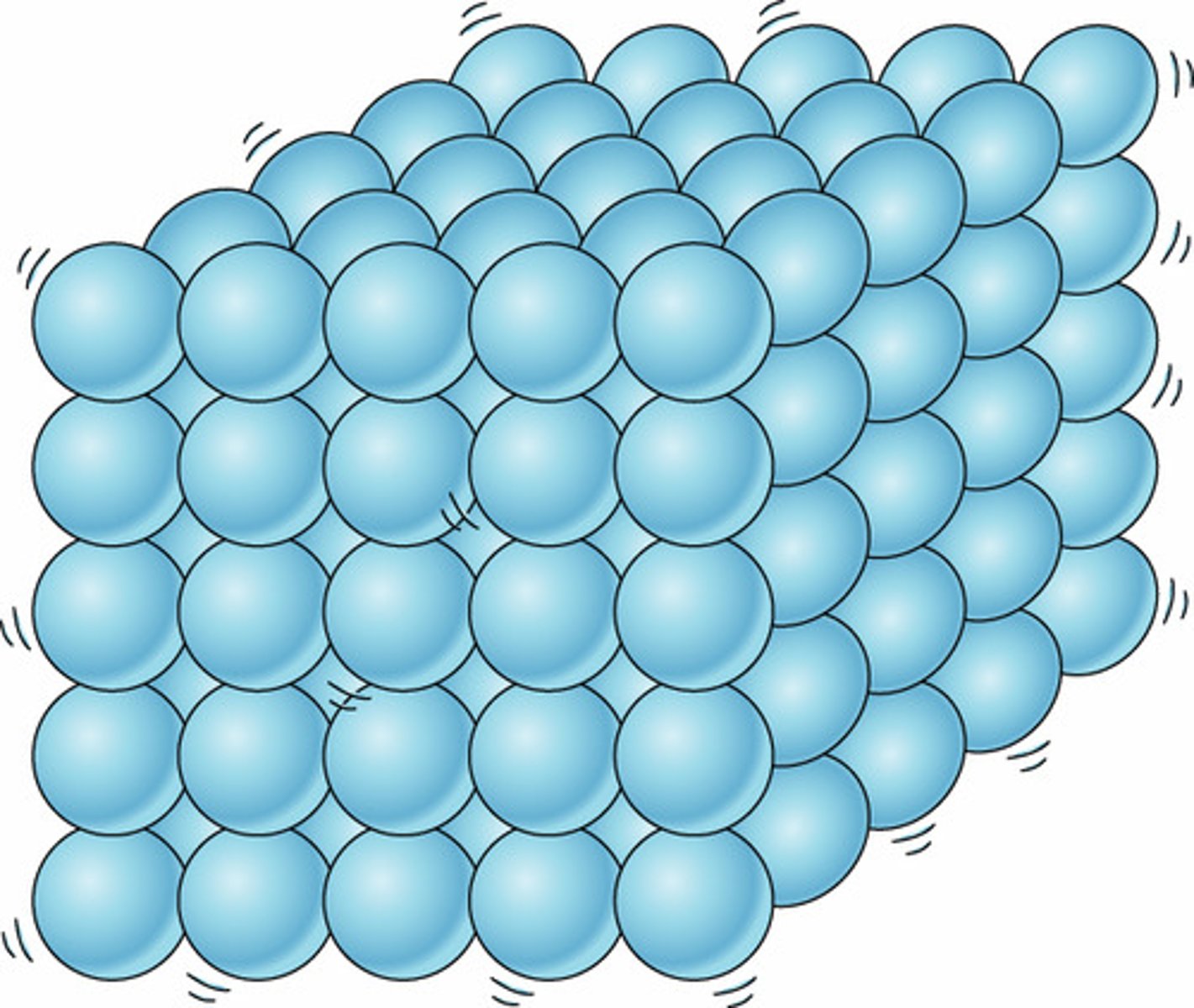
Solid state
Regular arrangement of particles, which are very close together and vibrate around fixed positions
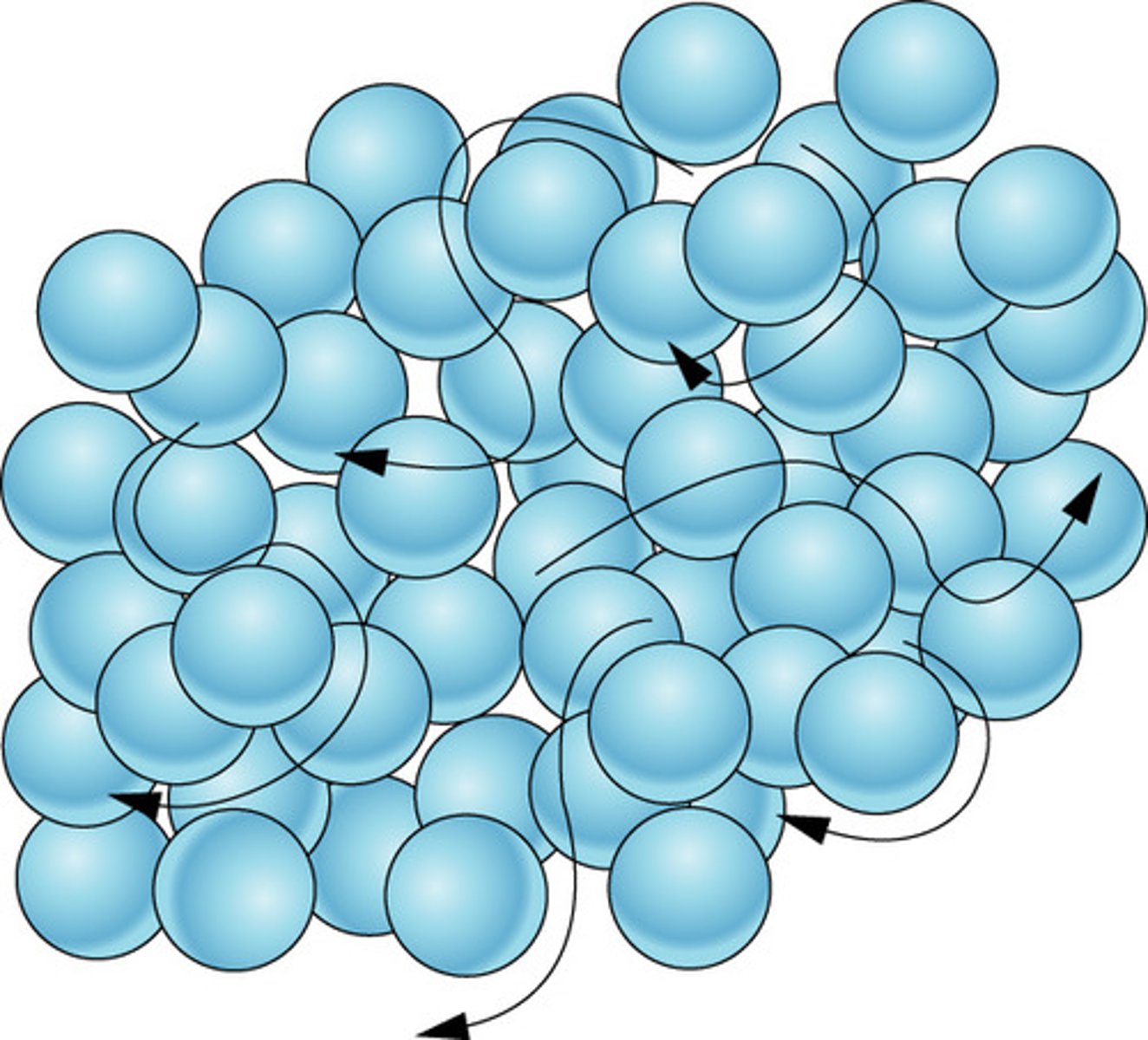
Liquid state, practice drawing!
Random arrangement of particles, which are close together and move around each other
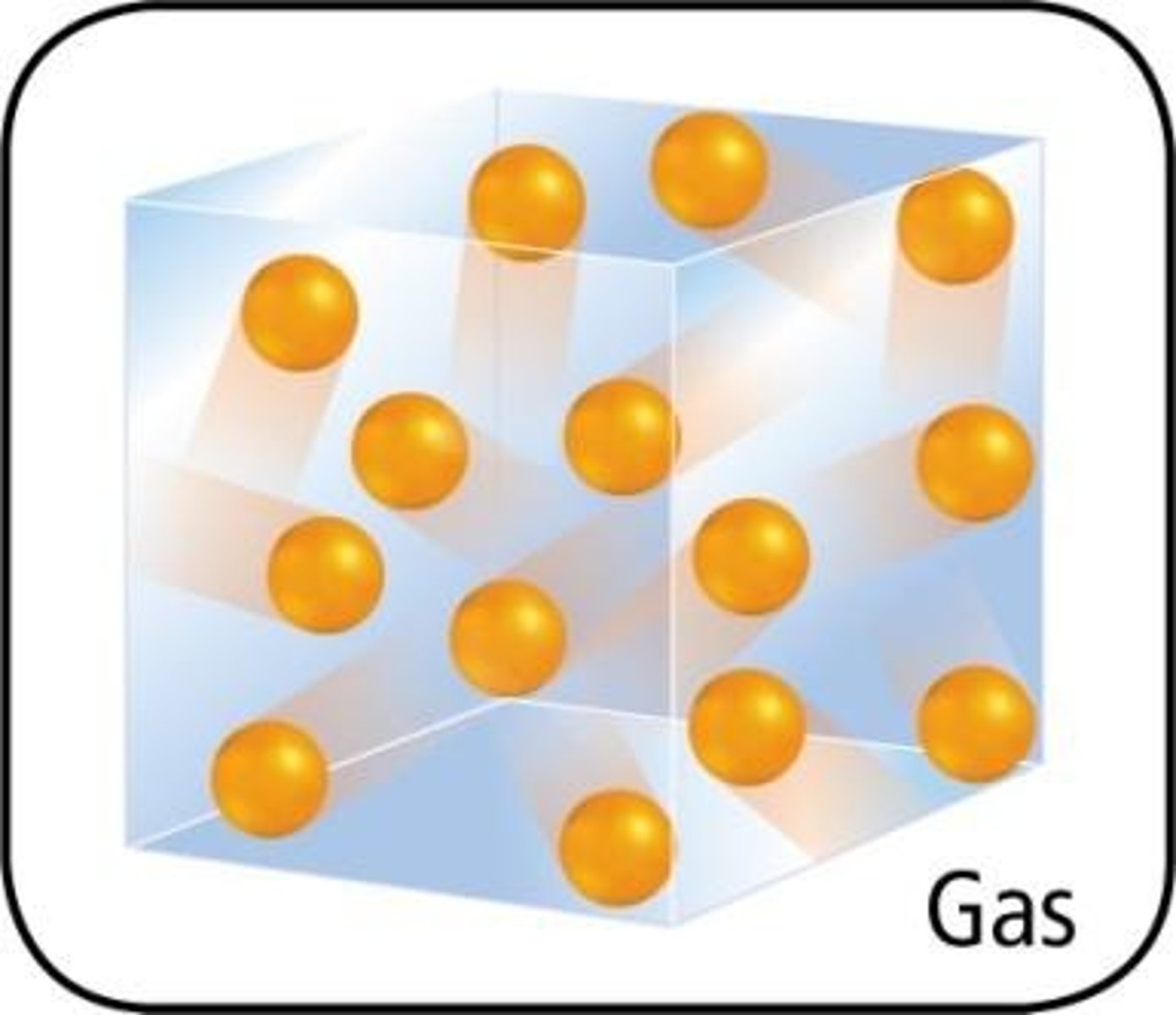
Gas state
Random arrangement of particles, which are far apart and move quickly in all directions
Particle model
Explains why some properties of a substance depend on its state
Why you cannot squash a liquid or solid, using the particle model
No space for particles to move into
Why a solid has a fixed shape and cannot flow, using the particle model
Particles vibrate around fixed positions and cannot move from place to place
Compromises that are made when drawing a 3D object on paper
You can only see parts of particles facing you and some are hidden
Type of 3D diagram on paper
Isometric
Physical change
Happens when a substance changes shape or state, or breaks into pieces, and where no new substances are made
Two examples of physical changes
Melting butter and cracking eggs
Three examples of reversable physical changes
Freezing juice to make an ice lolly, mixing sand with water and dissolving sugar in water
Chemical change
When new substances are made, the properties of which can be very different from those of the original substance
Two things you may observe during a chemical change
Colour change and / or gas given off, bubbles
Three examples of chemical changes
Cooking eggs or other food, steel rusting and an acid reacting with an alkali to make a salt and water
Smallest atom
Helium
Diameter of helium atom
62 pm or 62 * 10 ^ - 12 m
Distance between two helium atoms in relation to diameter
≈ 55 x larger, making it difficult to draw gas particles to scale
Forces between particles
Forces of attraction between positive and negative charges, so electrostatic
The further apart particles are, forces become -
Weaker
What forces between particles are strongest in
Solids
What forces between particles are weakest in
Gases
Limitations of the particle model
Does not take into account forces between particles, size of particles and space between particles
Why the volume of a substance generally increases a little when it melts
Forces of attraction get weaker and particles move around each other
What atomic radii and bonds lengths are usually around
10 ^ - 10 m
What 'atom' is Greek for
Indivisible
John Dalton's contribution, 1803
Proposed all matter consists of indivisible tiny particles, atoms. Atoms of each element are different to one another because they have different masses and atoms combined in simple whole number ratios
Joseph John Thompson's contribution, 1897
Proposed a model where atoms were positively charged spheres with negatively charged electrons embedded within. Discovered electrons in his experiments of electric discharge in a high-vacuum cathode-ray tube
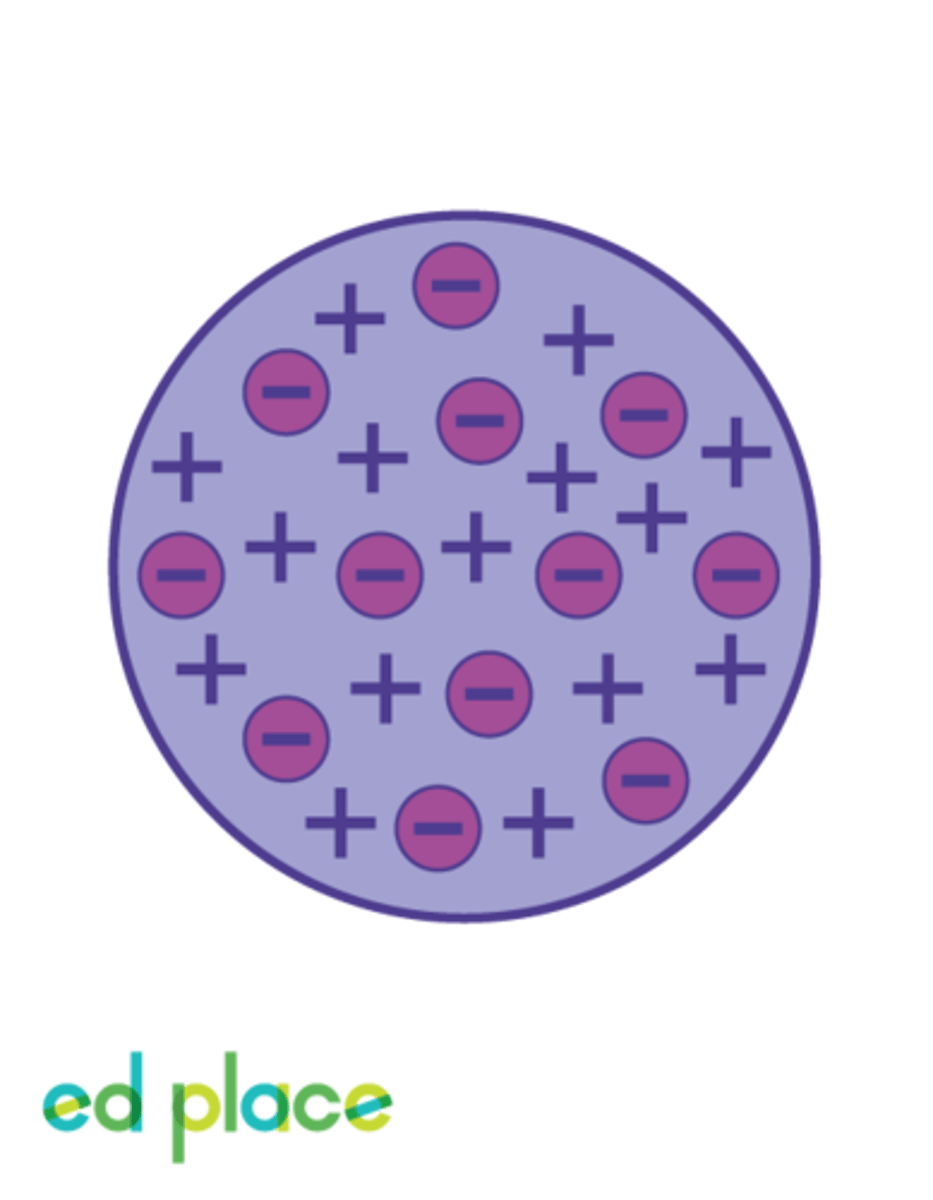
Plum-pudding model, devised by J.J. Thompson
Showing atomic structure, this theory held that the negatively charged electrons in an atom were floating - sometimes moving - in a sea of positive charge - the electrons being akin to plums in a bowl of pudding
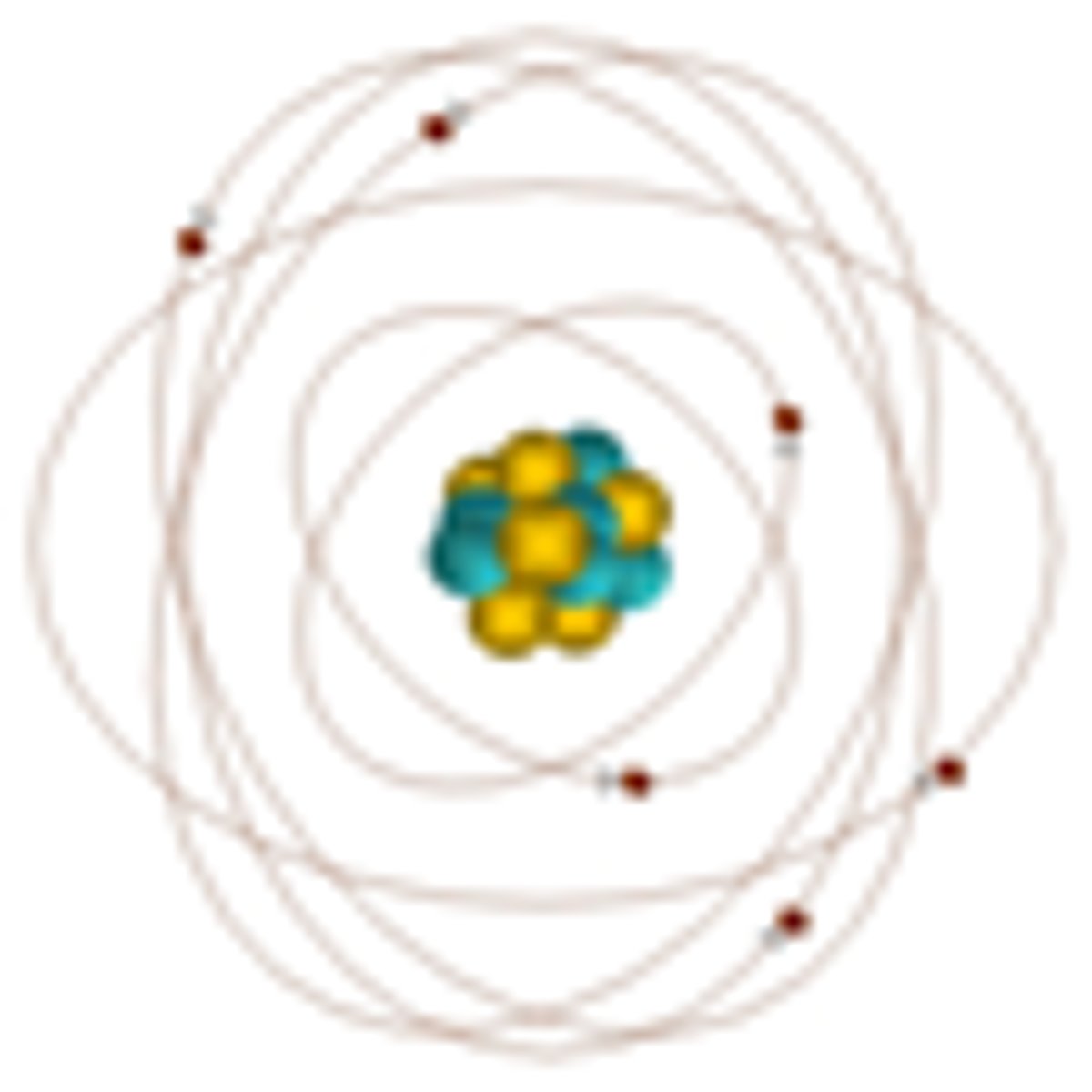
Ernest Rutherford's contribution, 1911
Famous experiment where most radioactive alpha particles that were fired at a thin sheet of gold passed straight through proved that atoms were mostly empty space, with a small, dense nucleus containing positive protons - negatively charged electrons orbited the nucleus. The change he suggested in 1911 showed a rapid change of ideas about the atom which had previously been based on Thompson's model
Who, when and where Ernest Rutherford's experiment was carried out by
Geiger and Marsden under the direction of Rutherford, at Manchester University in 1909
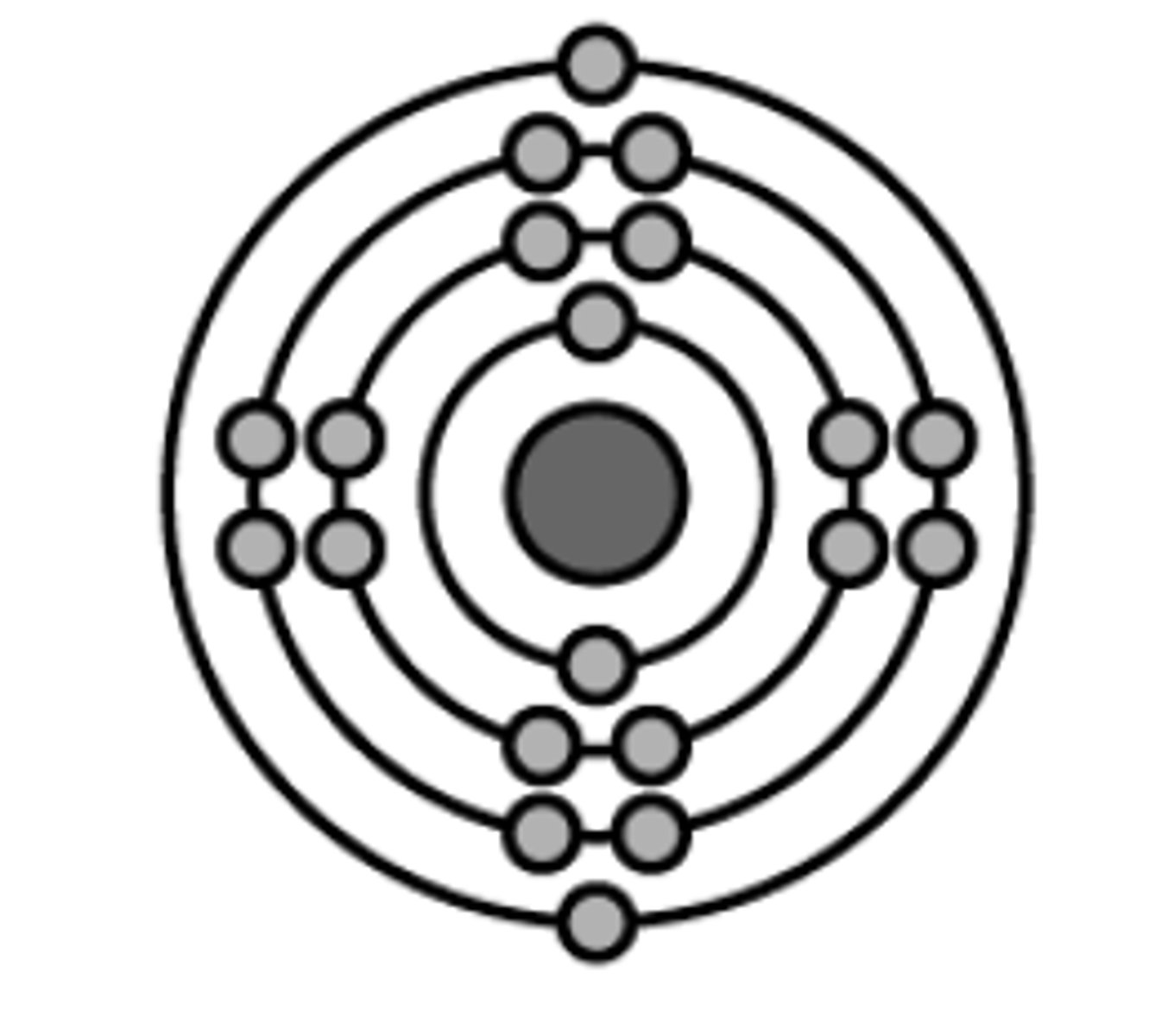
Niels Bohr's contribution, 1922
Suggested that electrons orbited the nucleus at different energy levels - in shells. Only electrons with a specific amount of energy could be found in each shell
Chemical symbol for an atom
Write some down!
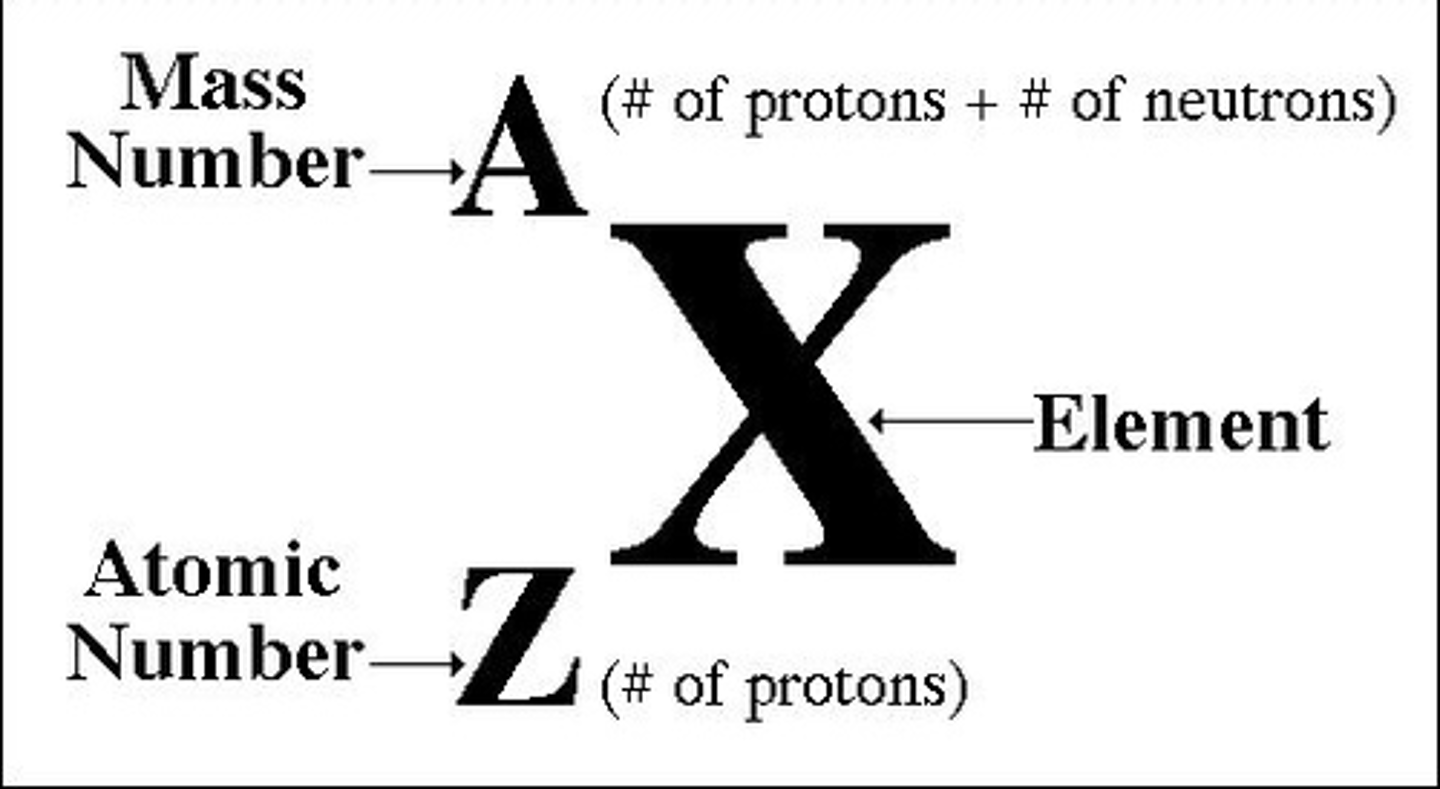
Atomic # (smallest #)
# protons in nucleus, also # electrons in an atom
Mass # (largest #)
Total # of protons and neutrons in nucleus
Isotopes of an element
Atoms with same # of protons and electrons, but different # of neutrons
What an isotope means for a chemical symbol
Same atomic #, but different mass #
All three isotopes of hydrogen
H{1, 1} / hydrogen-1, deuterium / heavy water is H{1, 2} / hydrogen-2 and tritium is H{1, 3} / hydrogen-3 (curly-bracket chemical notation is mine)
Periodic table
Chart of the elements showing the repeating pattern of their properties