Lab C: Separation using the Extraction Technique
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Miscible definition and example
-not suitable for extraction
-Miscible pairs include acetone and water in which you must have two immiscible liquids to carry out a liquid-liquid extraction
How do solvents separate in an extraction schematic?
-More dense solvent, sinks to the bottom and less dense solvent, floats to the top
What’s the density of water?
1.00 g/cm3
Whats the density of tBME?
0.74 g/cm3
What organic compounds are used in Lab C?
Benzoic acid, 2-naphthol, and 1,4 dimethoxybenzene
What functional group does Benzoic acid have and is it weakly or strongly acidic?
-carboxylic acid
-strongly acidic
What functional group does 2-naphthol have and is it weakly or strongly acidic?
-phenol group
-weakly acidic
What functional group does 1,4 dimethoxybenzene have and is it weakly or strongly acidic?
-ether
-neutral compound
Flow chart of Separation of a Ternary mixture by extraction
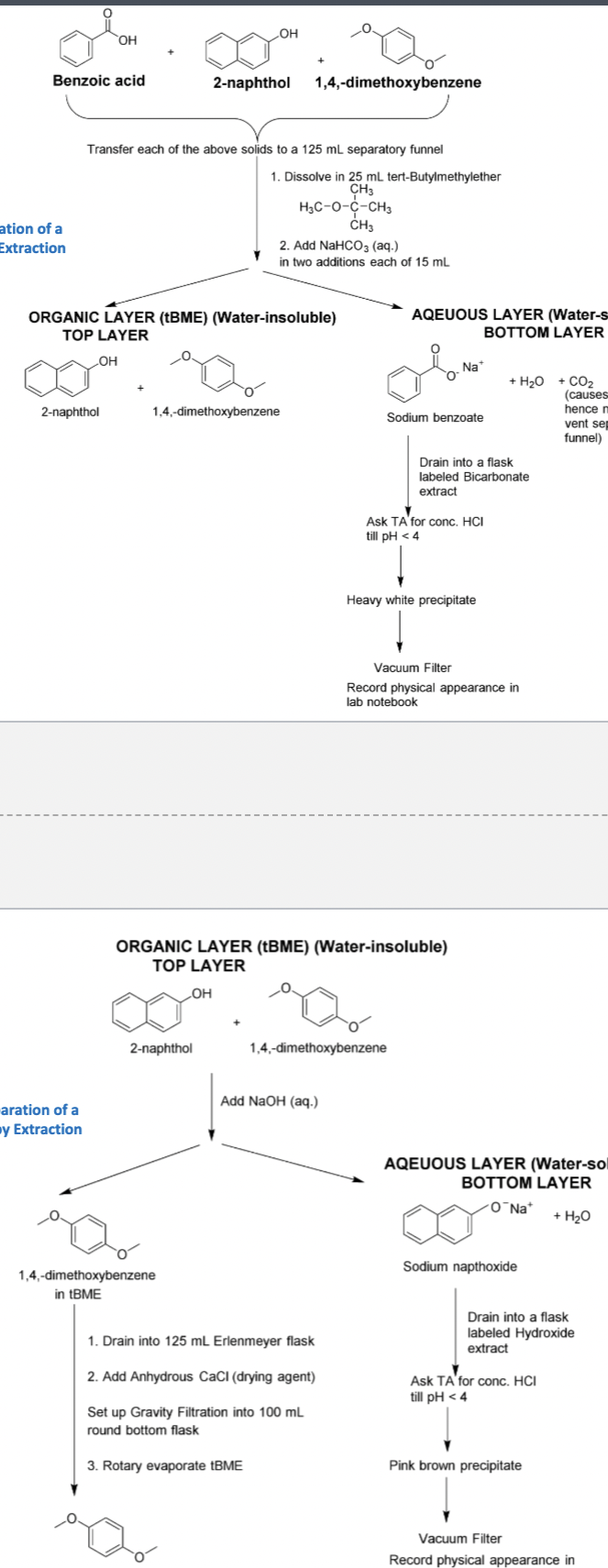
Acid Base reaction scheme of Benzoic acid and NaCHO3
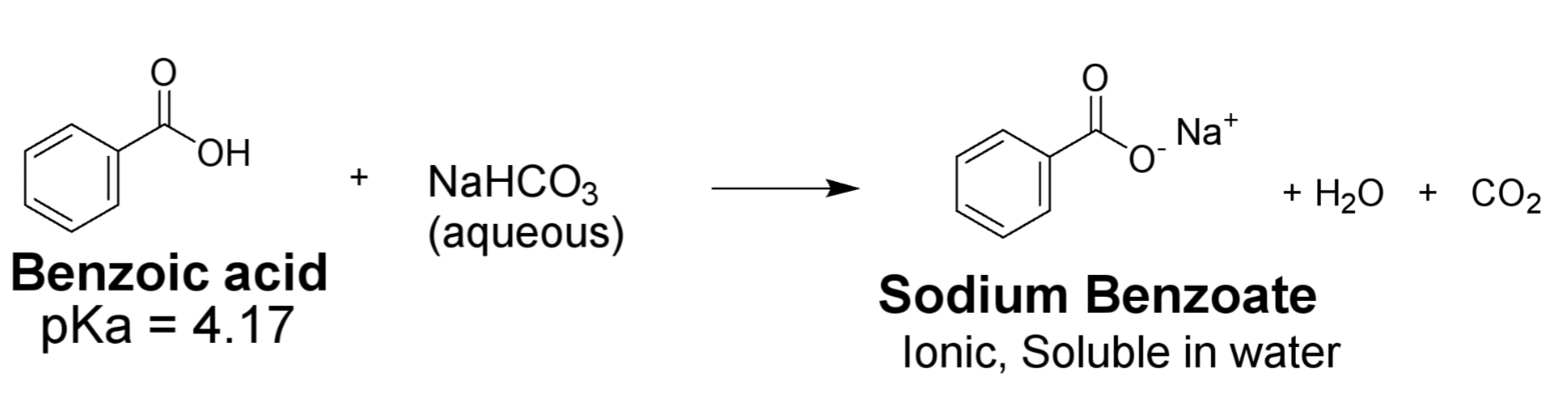
Acid Base reaction scheme of 2-naphthol and NaOH
How do compounds separate during an extraction technique?
-Compounds partition into two layers based on solubilities
-Salts, ionic species will be in the Aqueous layer
-Organic compounds will be in the Organic layer
What are the forces that control solubilty?
Van der Waals forces, Dipole-Dipole forces, Hydrogen bonding
Equation for partition coefficient

Theory of extraction
It is better to do several smaller extractions than one big extraction, to maximize the amount of material extracted and increase theoretical recovery
Equation for % recovery

Schematic for Separatory funnel
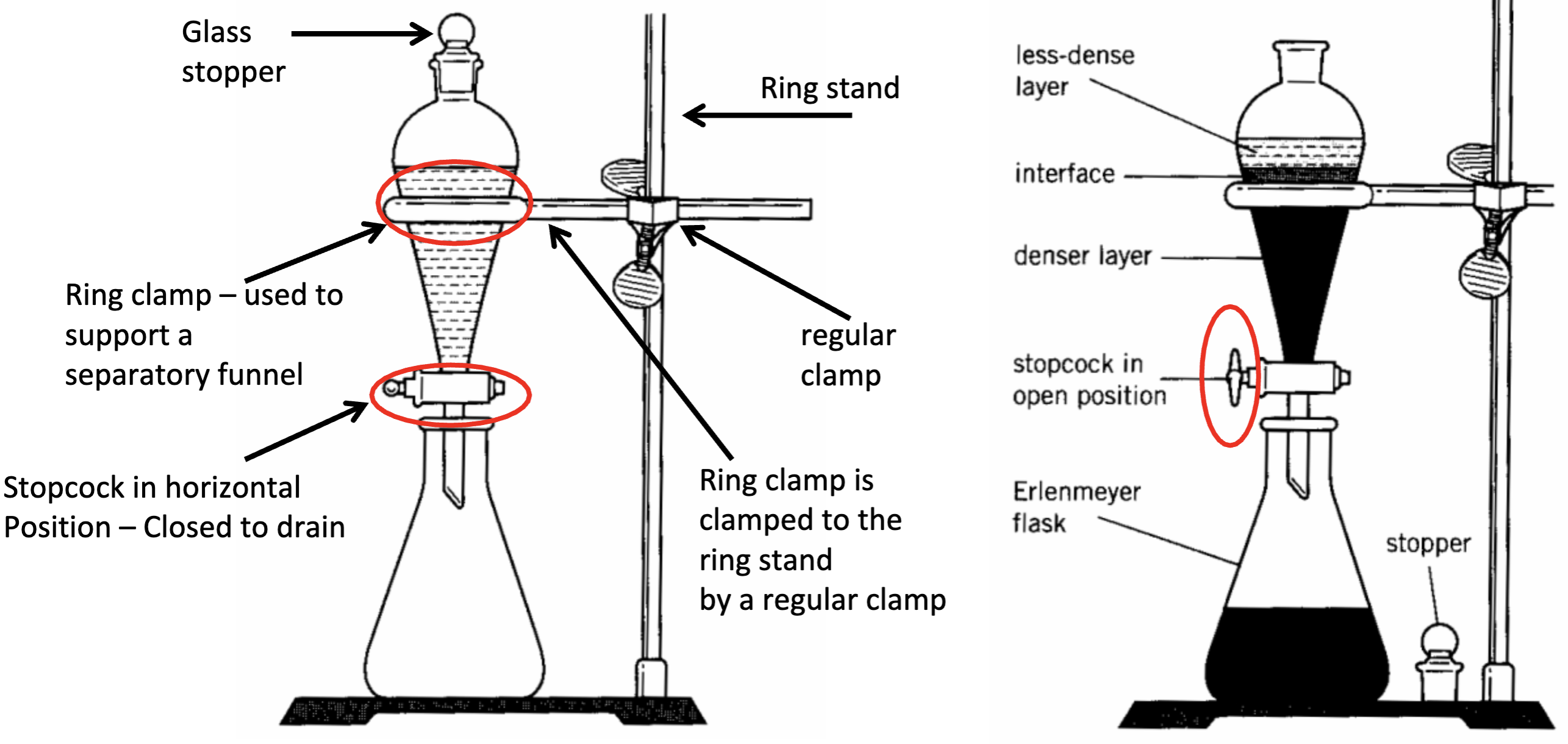
What should you do before draining the solution in the separatory funnel and what will happen if you don’t?
Remove the glass stopper because if you don’t the solution won’t drain
What should you use to add liquid solutions into a separatory funnel?
Pyrex funnel
What’s the density of dichloromethane?
1.33 g/cm3
Why should you vent your funnel while shaking?
Because pressure builds up in the funnel due to the formation of the CO2 gas
What are drying agents?
-They are anhydrous salts that combine with the water that is mixed in with your product and retains it as water of crystallization
Ex.) CaCl2
What happens when we use CaCl2 on our mixture?
-when all the water is bound with the salt we gravity filter the mixture
-the liquid devoid of water will pass through the gravity filter paper into the pre-weighed round bottom flask, so the hydrated salts stay on the filter paper
What should you do to get rid of the clumps from the drying agent?
-Add enough of the drying agents so it can become free floating
Gravity Filtration Schematic Set Up
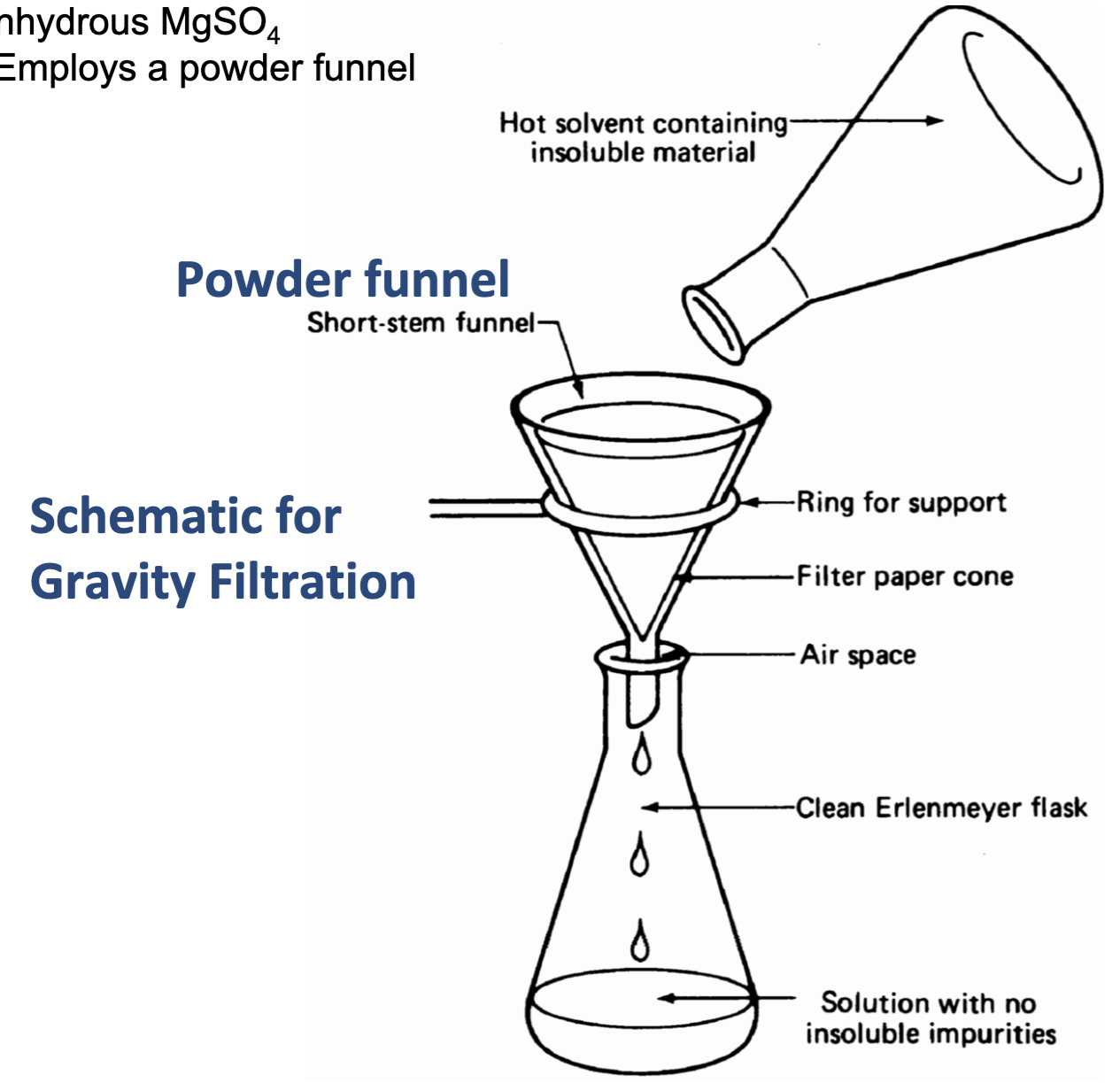
When should we use Gravity Filtration?
-when solute is mixed with a low boiling solvent (dichloromethane-CH2Cl2, diethyl ether)
-used to filter drying agents like CaCl2
-uses a powder funnel
When should we use Vacuum Filtration?
-used when solute is mixed with high boiling organic solvents (acetone, water)
-uses a buchner funnel
Why can’t we use Vacuum Filtration for low boiling solvents?
Because the solvent will evaporate during the filtration and contaminate the product crystals with impurities
Rotary Evaporator Schematic
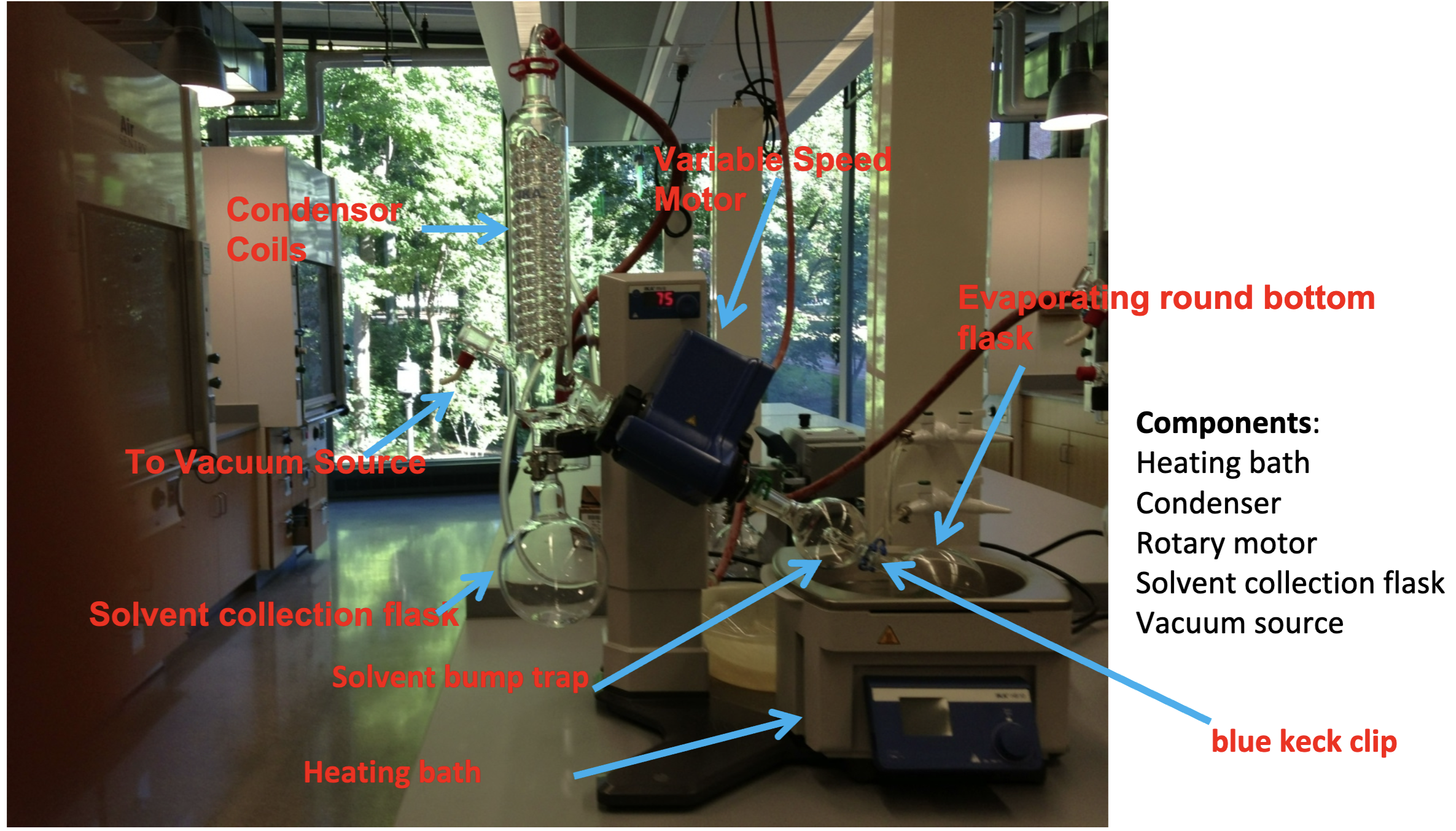
What does a roto vap do?
-it spins the round bottom flask to increase the surface area covered by the solvent
-applying the vacuum reduces the boiling point of the solvent being removed
-spinning flask prevents solvent from bumping
What happens if you fill the round bottom flask more than half full?
The solvent and product will spill over into the solvent bump trap
What happens if you use a broken blue keck clip?
The round bottom flask will slip into the hot water bath
What are emulsions?
A suspension of immiscible droplets of one liquid in another liquid, which creates a loss of two phases
How do emulsions form?
Shaking the separatory funnel too vigorously, even though shaking too gentle may not give effective mixing of the two phases
How to get rid of an emulsion?
-Let the separatory funnel sit undisturbed in the ring clamp for a few minutes
-stir the emulsion interface with a glass rod
-add a little salt
What type of base can deprotonate 2-naphthol and what does it form?
A strong base like NaOH to form a water-soluble salt, sodium napthoxide
What type of base can deprotonate benzoic acid and what does it form?
A weak base like NaCHO3 and it forms sodium benzoate
How can we remove benzoic acid from the organic layer into the aqueous layer?
We can use the aqueous solution of the weak base NaCHO3, leaving the 2-naphthol and 1,4 dimethoxybenzene in the organic layer
How can we recover the benzoic acid from the aqueous layer?
Add a strong acid like HCl so that the sodium salt can be protonated to regenerate benzoic acid
How can we separate 2-naphthol from 1,4 dimethoxybenzene in the organic layer (tBME)?
-Use a strong base like NaOH then a strong acid like HCl to regenerate
How can we separate 1,4 dimethoxybenzene?
-drain it from the separatory funnel and treat with an anhydrous drying agent, do a gravity filtration, then do a roto vap to remove tBME solvent
Which is more polar sodium benzoate or benzoic acid?
Sodium benzoate
Why do you carry out a second extraction with 10% sodium bicarbonate solution?
Because this ensures we’ve extracted all the benzoic acid from the organic layer into the aqueous layer
What is the purpose of using a 10% sodium hydroxide solution in your extraction lab?
The purpose is to act as a strong base to deprotonate the weak acid, 2-naphthol to form the sodium salt 2-naphthoxide.