21 Non-metals
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
General physical properties of non metals
low m.p. and b.p.
Can be solid liquid or gas at room temperature
Poor conductors of electricity and heat (except graphite)
When solids they are weak, brittle and dull
They have low densities
Specific properties of hydrogen
Colorless, odorless and tasteless gas at room temperature
Virtually insoluble in water
The lightest known element, less dense that air
Specific properties of chlorine
Yellow-green, poisonous gas with a strong odour
Moderately soluble in water
More dense than air
Specific properties of oxygen
Colorless, odourless and tasteless gas at room temperature
Slightly soluble in water
Slightly denser than air
Specific properties of carbon
Has two main allotropes: Diamond and graphite
*see solid structure
Specific properties of Sulfur
Solid at room temperature
Yellow in colour
Specific properties of Nitrogen
Colourless, odourless and tasteless gas at room temperature
Virtually insoluble in water
Slightly less dense than air
Chemical properties of non metals
Ionizes when they react with metals to gain electrons forming anions
Non-metal behaves like an oxidizing agent since it removes electrons from the metal
Results in the formation of organic compounds
Reactions of non metals and metals (general)
Non metal - oxidizing agent
Metal - reducing agent
Reactions of non metals and oxygen (general)
Oxygen - oxidizing agent
Non metal - reducing agent
Most non metal oxides are … but some are …
Acidic
Basic
Reaction of hydrogen and metal
Produces metal hydrides
Reaction of hydrogen with oxygen
Burns with very pale blue flame to produce water as steam
reaction of chlorine with metal
Produces metal chlorides
reaction of oxygen with metal
Produces metal oxides
Reaction of carbon with oxygen
Burns to produce either carbon monoxide - if there’s a limited supply of oxygen, or carbon dioxide - if there’s is a plentiful supply of oxygen
reaction of sulfur with metal
Produces metal sulfides
Reaction of sulfur with oxygen
Burns with a blue flame to form sulfur dioxide
reaction of nitrogen with metal
Produces metal nitrides
Reaction of nitrogen with oxygen
Reacts with oxygen if temperature is high enough to form nitrogen monoxide
Non metals as oxidizing and reducing agents
Unless reaction with oxygen, non metals are usually oxidizing agents
Some however, can also act as reducing agents : hydrogen, carbon, sulfur, nitrogen
How to determine which method should be used to prepare a gas
Consider:
solubility of gas in water
Reactivity of gas with different drying agents -
Density of the gas compared to density of air
How to know if a gas is more or less dense than air
if RMM/2 > 14 the gas is more dense than air, if RMM/2 < 14 the gas is less dense than air
If a gas is more dense than air what do u use to prepare it
Downward delivery ( upward displacement)
If a gas is more less than air what do u use to prepare it
Upward delivery (downward displacement)
In order to be collected over water, the gas must be
Insoluble / sparingly soluble in water
Unreactive with water
Draw a diagram of apparatus used to prepare oxygen
Draw a diagram of apparatus used to prepare carbon dioxide
Draw a diagram of apparatus used to prepare Ammonia
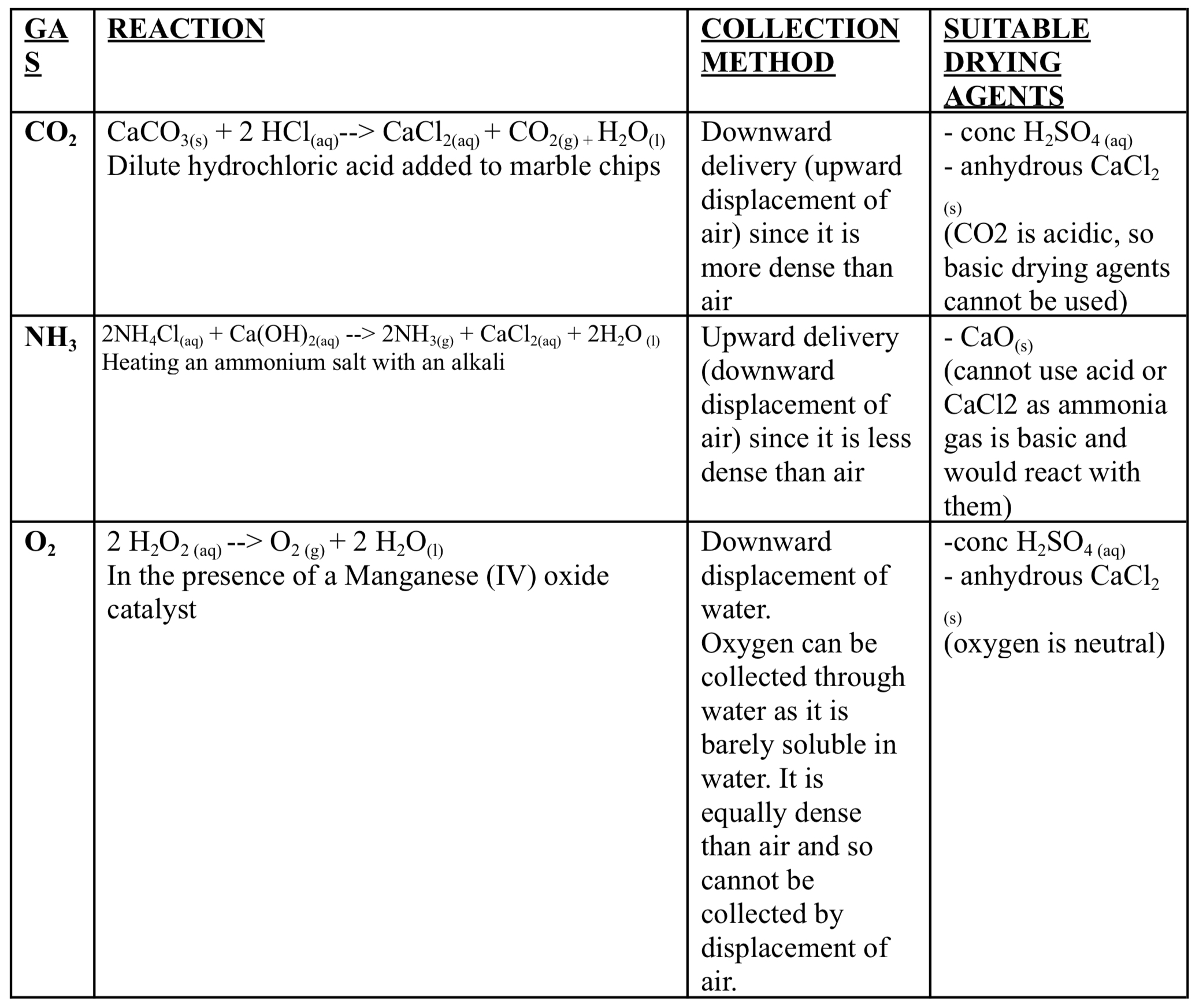
Summary of preparation of gases table
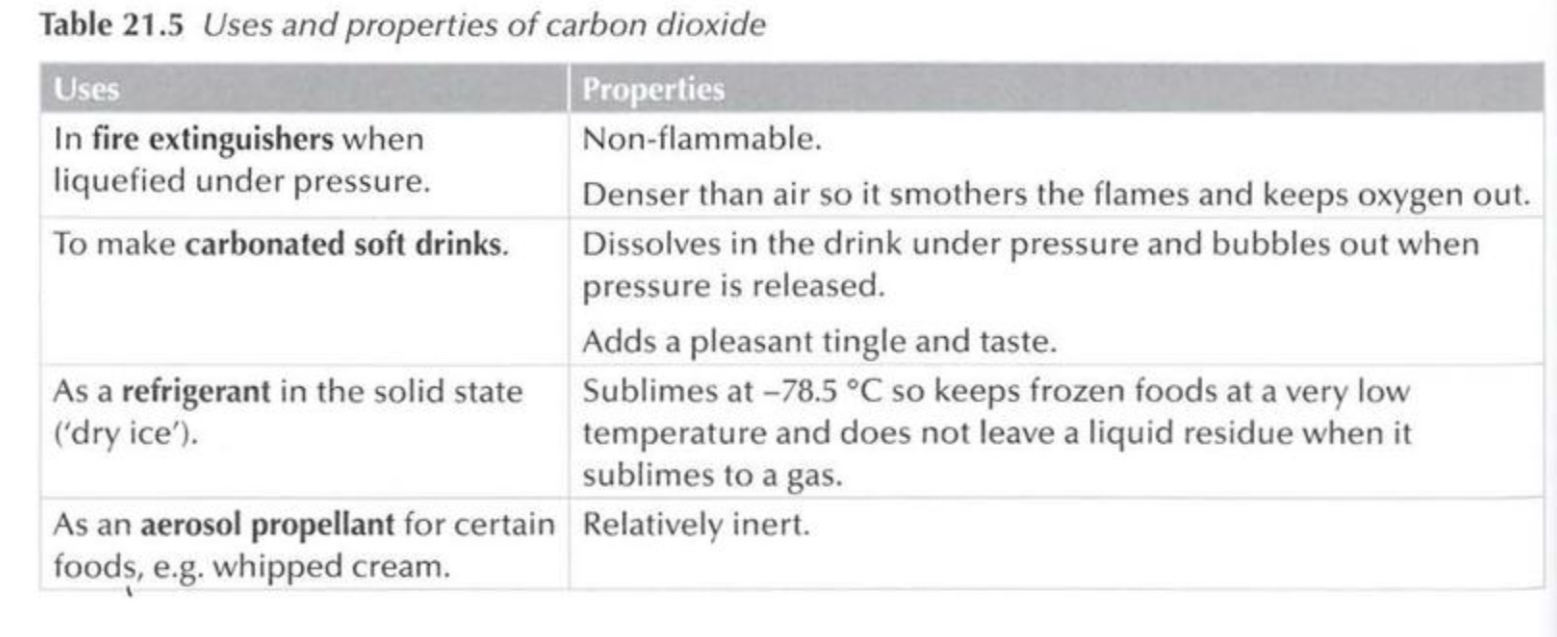
Uses and properties of carbon dioxide
Uses of oxygen
essential for living organisms to carry out aerobic respiration to release energy

Uses of non metals and their compounds
Harmful effects of non metals and their compounds
Disposal of waste containing plastic