Chap 9D - Acid-base equilibria
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
Explain how ocean maintains stability
When a small amount of acid is added, CO3^2- (aq) + H+ (aq) → HCO3- (aq)
Large reservoir of CO3^2- remove the added H* ions and pH remains almost constant
When a small amount of base is added, HCO3- (aq) + OH- (aq) → CO3^20 (aq) + H2O(i)
Large reservoir of HCO3- remove the added OH- lons and pH remains almost constant
This system helps maintain the ocean's pH around 8.1, which is slightly basic and optimal for marine life
Describe ocean acidification
Human activities have increased atmospheric CO2
More CO2 dissolves in the ocean, forming more carbonic acid
Carbonic acid dissociates, releasing more H+ ions, which lowers the ocean's pH
The overall rapid and large increase in H* ions can overwhelm the ocean's natural buffering system and reduce the concentration of carbonate ions
Calcifying organisms struggle to build and maintain their shells and skeletons, leading to weaker structures and greater vulnerability
Describe other uses of buffer
In industrial processes such as electroplating and manufacture of dyes, photographic materials
In chemical analysis and for the calibration of pH meters
In agriculture to maintain the pH of soll to optimise plant growth
In bacteriological research to maintain the pH of culture medla used for the growth of bacteria
In intravenous injections so as not to change the pH of blood
Describe chemical reactions involved in ocean acidification
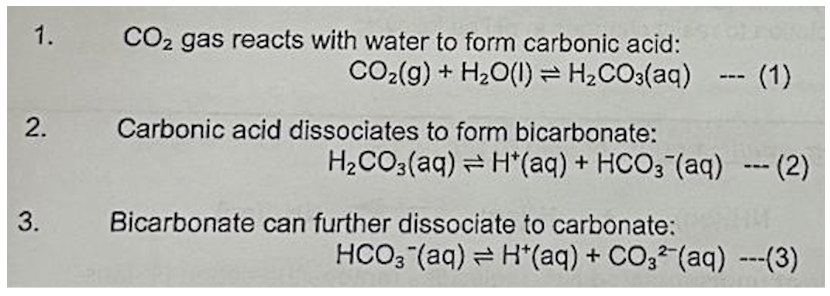
Using chemical eqautions, explain ocean acidification
As [CO2(g)] increases, by Le Chatelier's Principle, the position of equilibrium in equation (1) shifts to the right, causing [H2CO3] to increase -> position of equilibrium in equation (2) to shift to the right, causing [HCO3-] to increase
As [HCO3-] increases, the position of equilibrium in equation (3) shifts to the right, leading to an increased [H+] -> causes the pH of seawater to decrease -> ocean acidification
Since the Industrial Revolution, the average pH of ocean surface waters has decreased by about 0.1 units, representing a 30% increase in acidity