Chap 5A - Enthalpy
1/24
Earn XP
Description and Tags
Up to election affinity
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Draw basic energy level and profile diagrams of endo and eco reactions
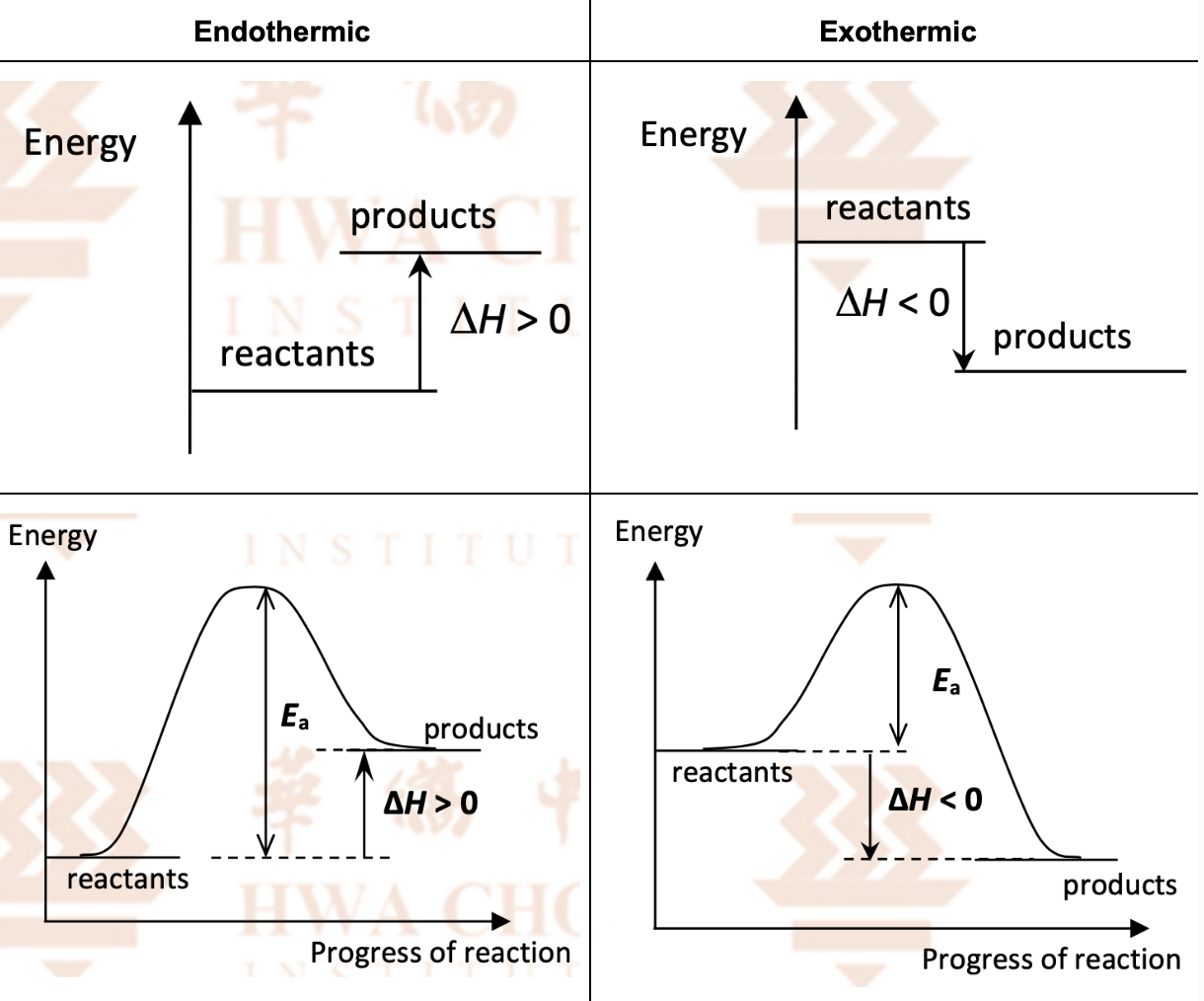
Compare endo and eco reactions with examples
Endothermic | Exothermic |
The products have higher energy content than the reactants | The products have lower energy content than the reactants |
|
|
|
|
Examples :
| Examples :
|
State the standard conditions and states
What is standard state
Standard state : a substance in its normal , most stable physical state at 298K and 1 Bar
Elements in their standard state under standard conditions are assigned 0 enthalpy
To compare energy changes, need to specify conditions under which the reaction was performed
What is the physical states of substances under standard conditions
Solids (majority)
Liquids
Mercury, Br, Gallium, Cesium, Francium
Diatomic gases - O2, H2, CI2
Monoatomic gasses - noble gases
Define enthalpy
Enthalpy (Def.): Total heat content of a substance but it cannot be measured directly
State the characteristics of enthalpy
H of a particular reaction (Hreaction or Hr) is associated with a balanced chemical equation
It refers to the enthalpy change for the stoichiometric molar amounts of reactants and products as indicated in the balanced chemical equation
H is directly proportional to the molar amounts of reactants
H of a reverse reaction is exact in magnitude but opposite in sign to the H of the forward reaction
H changes with the physical states (solid, liquid and gaseous) of the reactants and products
Chemical equations must be accompanied by state symbols
Define and describe SEC of reaction
The enthalpy change when molar quantities of reactants as specified by the chemical equation react to form products at 1 bar and 298 K (standard conditions)
This is the heat absorbed or evolved, usually associated with breaking and forming of chemical bonds.
H = (Total enthalpy of products) – (Total enthalpy of reactants)
Define SEC of formation
(Def.): The enthalpy change when one mole of a pure substance is formed from its constituent elements in their standard states, under standard conditions of 298K and 1 bar
Coefficient of HI MUST be 1
State some important considerations of SEC of formation
If an element has allotropes , the more stable allotrope is chosen (Eg. Graphite (not diamond) is chosen as the standard state for carbon)
From the definition, the enthalpy change of formation of elements in their standard state is 0 kJ mol–1
Hf⦵ of a compound represents the heat transferred to or from the surroundings when chemical bonds in the elements are broken and new bonds are formed in the compound
Measures the stability of the compound relative to its constituent elements
If positive (endo) : compound is energetically less stable than its constituent elements -> greater likelihood for compound to decompose into its constituent elements
More negative (more exo) -> more stable
Hf⦵ can be used for calculation of enthalpy change of various reactions
Enthalpy changes of formation are often theoretical
Some reactions may not take place in practice
Define SEC of combustion, what it is useful for and its considerations
(Def.): The enthalpy change when 1 mole of a substance is completely burnt in excess oxygen under standard conditions of 298K and 1 bar
Useful for:
Identification of substances with large Hc values as sources of fuel
Determination of calorific value of foodstuffs
Calculation of enthalpy change of various reactions
Note:
Always exothermic
Applies to complete combustion of 1 mole of substance
For carbon, the combustion product is CO2 and NOT CO
Define SEC of neutralisation
(Def.): The enthalpy change when 1 mole of water is formed when an acid neutralises a base and is carried out in an infinitely dilute aqueous solution under standard conditions of 298K and 1 bar
Hneut⦵ is always negative as heat is always evolved due to formation of bonds between H+ and OH- to form H2O during neutralisation
Compare SEC of neutralisation for strong acids and bases VS weak acids and bases
Almost the same for all strong acids and bases (--57.0 mol-1)
Strong acids and strong bases ionise completely in dilute aqueous solution
Reaction between them is effectively the reaction between aqueous H+ ions and OH– ions
Slightly less exothermic than --57.0 mol-1 for weak acid and bases
The neutralisation of a weak acid and a strong base will involve an additional endothermic process – ionisation of the weak acid
Part of the energy released during the neutralisation process is absorbed to break up the undissociated acid molecules, resulting in a less exothermic Hneut⦵
Define SEC of atomisation and Hatom of a compound
(Def.): The enthalpy change when 1 mole of isolated gaseous atoms are produced from the element in its standard state under standard conditions of 298K and 1 bar
(Def.): Hatom m for a compound is the energy required to convert 1 mole of the compound into gaseous atoms at 298 K and 1 bar
H Ꝋ atom values are always positive since heat is always absorbed as the bonds in the elements must first be broken before atoms can be released (endo)
Define SEC of hydration
(Def.): The enthalpy change when one mole of gaseous ions is dissolved in a large amount of water under standard conditions of 298 K and 1 bar
State some considerations of SEC of hydration
Always negative (exo):
For both cations and anions because the gaseous ions are always attracted to the oppositely charged ends of the polar water molecules, leading to the formation of ion-dipole interactions
Heat is evolved during the formation of ion-dipole interactions in the hydration process
The ions are dissolved in such a large amount of water that further addition of water produces no further heat changes
The higher the charge-radius ratio (charge density), the stronger is the ion-dipole interactions formed and the hydration energy released is more exothermic
Define SEC of solution
(Def.): The enthalpy change when one mole of a solute is completely dissolved in a solvent to form an infinitely dilute solution under standard conditions of 298K and 1 bar
The solute is usually an ionic compound while the solvent is usually water
Can be either positive or negative
Highly positive : salt is likely insoluble
Negative : salt is likely soluble
Define lattice energy
Definition : The energy released when one mole of an ionic solid is formed from its isolated gaseous ions from an infinite distance apart
Always negative as heat is evolved in forming electrostatic forces of attraction between oppositely charged ions
X+ (g) + Y+ (g) -> XY (s)
Measure of the strength of ionic bonding and the stability of the ionic compounds
The more exothermic the lattice energy, the stronger the ionic bonding and the more stable the ionic compound
Describe the factors of lattice energy
Charge on the ions
The greater the ionic charges on ions, the stronger the electrostatic forces of attraction between the ions and hence lattice energy is more exothermic
Sizes of ions or inter-ionic distance
The smaller the ions, the closer they can approach each other in the solid crystal -> shorter inter-ionic distance -> electrostatic forces of attractions between ions are stronger and lattice energy is more exothermic
Crystal structure
The arrangement of ions in the crystal lattice will affect the attraction and repulsion between ions in the compound
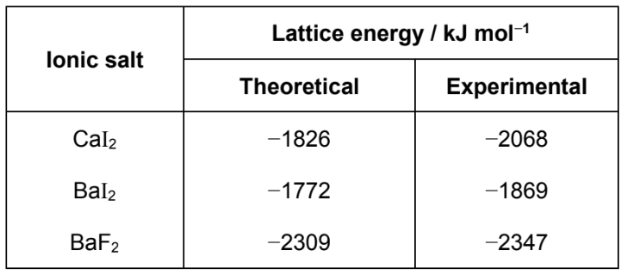
Compare difference in theoretical and experimental lattice energies of BaI2 and BaF2
Comparing between BaF2 and BaI2, I− has a larger ionic radius and hence a larger and more polarisable electron cloud
This leads to a larger extent of distortion of the electron cloud of in I−, which results in a more covalent character in BaI2
Hence, there is a bigger difference between the theoretical and experimental lattice energies of BaI2 compared to BaF2
Experimental lattice energy : refers to the value found from experimental results using the Born-Haber cycle
Theoretical lattice energy : refers to the value calculated based on a model which assumes that the compound is completely ionic
Define bond energy
(Def.): The energy absorbed when 1 mole of covalent bonds between 2 atoms in a gaseous molecule are broken
Always positive because energy is needed to pull the two atoms apart (endothermic process) to break the covalent bond
Why is bond energy defined with respect to molecules in the gaseous state?
If we were to define bond energy based on solids e.g. I2(s), the energy change for
I2(s) → 2I(g) would also include the energy needed to sublime the solid iodine. Hence, it
does not actually reflect the actual amount of energy that is needed to break the I−I
covalent bond
State the limitations of bond energies
Due to the reasons above, the enthalpy change of reaction calculated using bond energies might differ from the actual enthalpy change of the reaction
Bond energies are average values, and not specific to particular bonds in any molecule
Bond energies are only applicable to molecules in the gaseous state
Define electron affinity
(Def.): The enthalpy change when one mole of a gaseous atoms or negatively charged ions gain one mole of electrons
Compare the 1st EA and 2nd EA
1st EA
Usually negative (eco) as the effective nuclear charge of the atom leads to an attraction of the incoming electron
When the attraction is stronger, the energy given off is greater and hence E.A. is more negative
2nd and above EA
Always positive (endo) because energy is absorbed to overcome the electrostatic repulsion between the incoming electron and the anion