Ch. 21 Nuclear Chemistry
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Alpha decay
loss of an alpha-particle (helium nucleus)
42He
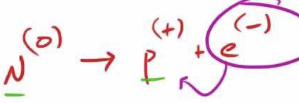
Beta decay
loss of beta-particle (high energy e-) from nucleus
0-1e
positron emission
positron=particle w/same mass of e- but opposite charge
01e
electron capture (k-capture)
adding an e- to a proton in the nucleus
result": proton —> neutron
Gamma emission
loss of gamma ray — very high-energy electromagnetic radiation
smaller nuclei (Z<20) have a stable neutron to proton ratio of
1:1
it takes more neutrons to stabilize the nucleus as nuclei are larger…
above the belt…
nuclei have too many neutrons
tend to decay by emitting beta particles
it takes more neutrons to stabilize the nucleus as nuclei are larger…
below the belt…
have too many protons
tend to become more stable by positron emission or electron capture
There are no stable nuclei with an atomic number greater than…
83
nuclei with such large numbers tend to decay by alpha emission
Nuclei with an (even or odd) # of protons and neutrons tend to be more stable
even
Nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons tend to be (more/less) stable
more stable
all nuclear decay is a _____ order process
first order
binding energy (BE)
energy released when nucleons combine to form a nucleus
depends on mass defect
Mass defect (Δm)
difference between mass (in kg) of a stable nucleus and the individual nucleons that comprise it
E=(Δm)c2