Particle model of matter
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What is the arrangment of particles in a solid?
Very close together
Regular patter
Vibrate but do not move
What is the arrangment of particles in a liquid?
Close together
Not in a regular pattern
Can move around eachother
What is the arrangment of particles in a gas?
Very far apart
Not in a pattern
Move very rapidly
What is density?
The density of a material tells us the mass for a given volume
(How much mass is packed into its volume)
What is mass?
Mass is the amount of matter or substance that makes up an object
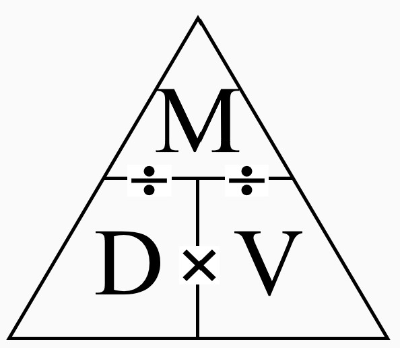
How is density calculated?
P = M / V
Density(Kg/m3) = mass(Kg) / volume(m3)
What are the densitys of solids,liquids and gases
Solids have a high density as their particles are closely packed together (a lot of mass)
Liquids have a high density as their particles are close together (a lot of mass)
Gases had a low density as their particles are very far apart (a low mass)
Why is polystrene a solid with a low density?
Has open structure and is fulled with air spaces
What are regular objects?
Objects that have dimensions easy to measure
What are irregluar objects?
Objects with dimensions that can not easily be measured
Required pratical 5: density of regular objects
First, find the mass of your regular object using a balance
Then work out the volume of the cube, using a ruler to measure the length of the side
The volume equals the length of the side cubed so times you measurement by three
Divide the mass and volume to find the density
Required pratical 5: density of irregular objects
Find the mass of the irregular object using a balance
Fill a eureka can with water which has a spout which allows the water to flow out the can
Place the object into the water
This will cause the water to be displaced and flow out of the spout
Measure the volume of water that was displaced using a measuring cylinder
This volume is the same as the volume of the object
Calculate the density by dividing these two number
What is internal energy?
Internal energy is the energy stored in a system by the particles
The internal energy is the total kinetic energy and potential energy of all the particles that make up a system
How does the internal energy change in the changes of states in solids, liquids and gas
As particles in solids → liquids → gas movements increase in this order, there is a increase in kinetic and potential energy
This means if you heat a solid, we increase the internal energy as it turns to a liquid
If you change the states from gas → liquid → solid movement decrease as there is a decrease of kinetic energy and potential energy
If you cool a gas down, then we reduce the internal energy as the gas will change to a liquid Wha
What is sublimation?
A solid turning directly to a gas
What happens to mass when the states of matter change?
Mass is conserved as we are not adding or taking particles away
Are the changes of state physical changes or chemical changes?
Physical changes as if we reverse the change, the materials recover back to their original properties
Evaporation is when a liquid turns to a gas but…
only on the surface of a liquid
In this case, only the particles on the surface have enough energy to turn into gas
What is specific heat capacity?
The specific heat capacity of a substance is the amount of energy required to raise 1 kg of the substance by 1’c
What is the equation for specific heat capacity?
△E = m x c x △θ
Change in thermal energy(J) = mass(Kg) x specific heat capacity(J/kg ‘c) x temperature change (‘c)
What will happen in heating graphs?
When the substance heats, we increase the temperature as we increase the energy particles
When the substance starts to change state eg melts, the temperature stops increasing and stays constant
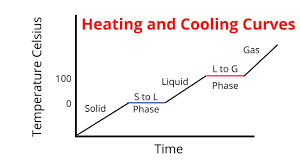
What will happen in cooling graphs?
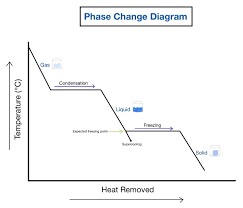
What happens to the force of attraction as we add energy?
It is weakening/breaking
What is the latent heat?
The energy needed for a substance to change state
Does the temperature change during the change of state?
No, the internal energy store is increased
What is the specific latent heat of a substance?
The specific latent heat is the amount of energy required to change the state of one kilogram of the substance with no change in temperature
What is the specific latent heat energy of fusion?
The energy required to change on kg of a substance from a solid to a liquid with no change in temperature
What is the specific latent heat of vaporisation?
The energy required to change 1 kg of a substance from a liquid to a vapour with no change in temperature
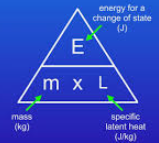
What calculation is used to find the energy for a change of state?
E = m x L
Energy for a change of state(J) = mass(Kg) x specific latent heat(J/kg)
What Whgas pressure due to?
The pressure of gas it due to the particles colliding with the walls of the container the gas is held in
What are the gas particles exerting when colliding with the walls of the container?
Pressure
How can we increase the pressure?
Increase the number of collisions
Increase the energy of each collision
How can we increase the energy of the particles in gas?
Increase the temperature
What is the temperature of a gas related to?
The average kinetic energy