inorganic chemistry Bio
1/66
Earn XP
Description and Tags
in the name of The Father, Son, and Holy Spirit 🙏
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
What is an atom
the smallest unit of matter
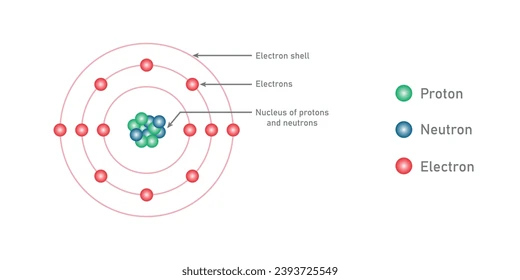
what are the compontent particles of an atom
Nucleus, Protons, Neutrons
what is a proton
A positive charge particle that is in the nucleus
What do protons determine
The chemical properties of an element
what are neutrons
A particle in the atom that have no charge
what are electrons
very tiny negatively charge particles that are attracted to protons and orbit the nucleus
what is an atomic number
the number of protons in the nucleus; it impacts the arrangement based on atomic number atomic number
What are Isotopes
Atoms of the same element that have a varying number of neutrons in the nucleus
even through istopes have varying numbers of neutrons in the nucleus…
isotopes of the same element have similar chemical properties, since those are determined by the number of protons
what is atomic mass
the average weight of atoms in that element -the number of protons and neutrons deteremine the mass
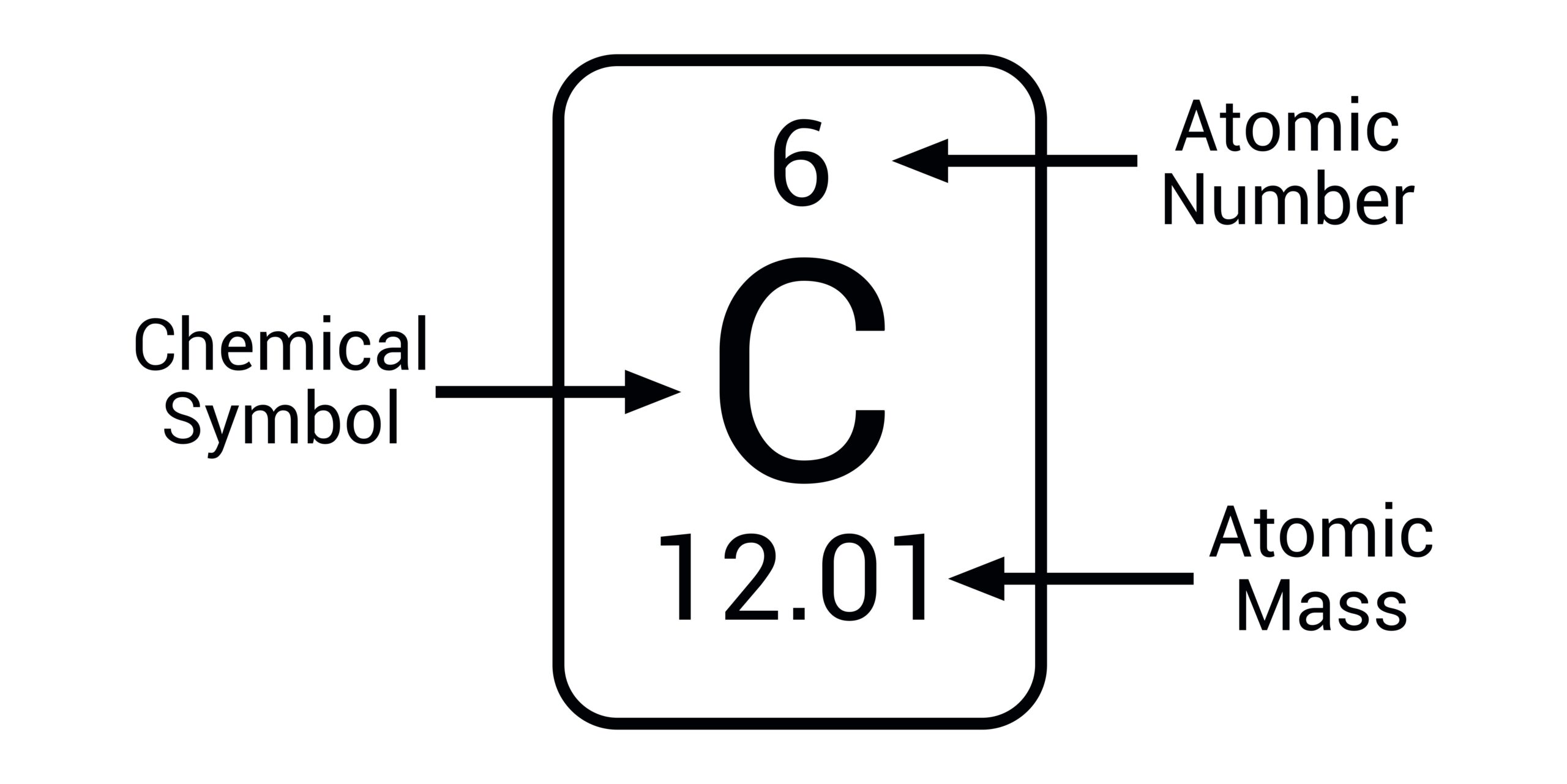
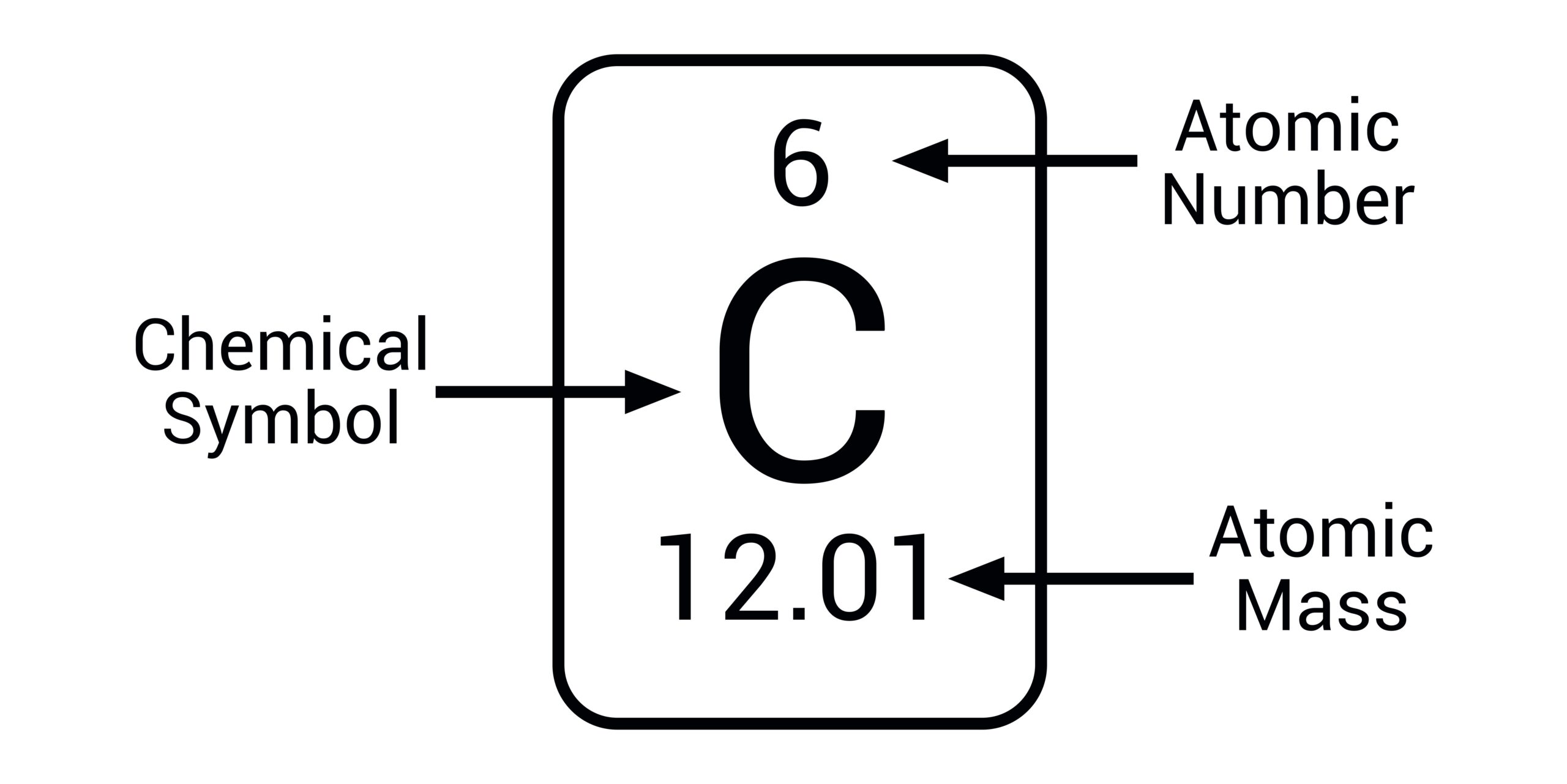
how do you read a chemical
top number is the atomic number and the second number (larger) is the average atomic mass
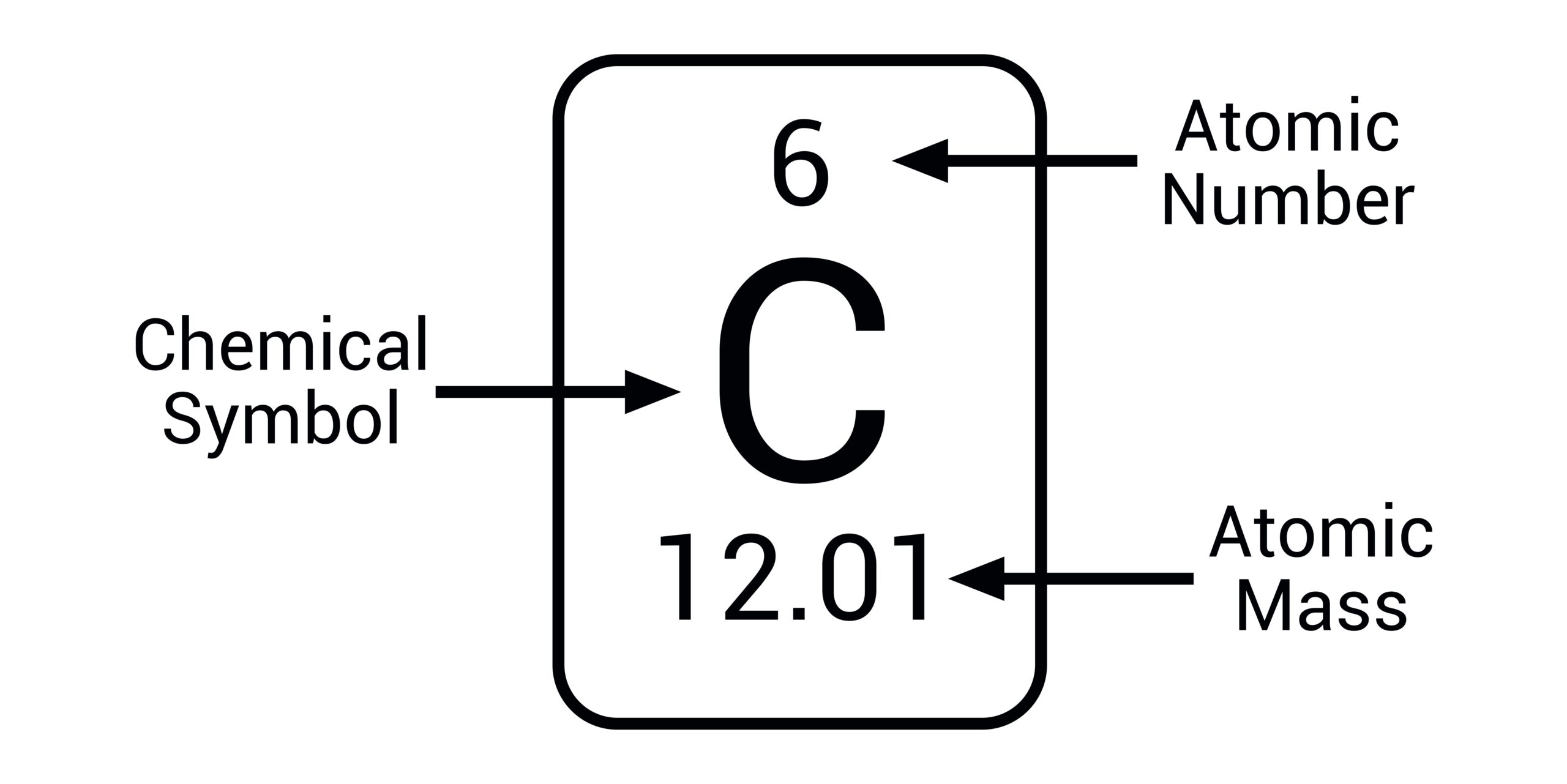
how do determine the number of particles in an atom
Protons=atoms Neutrons= Mass number - atomic number Electrons= atomic number - charge (Protons and electrons will be the same)
What is valence
the number and energy level of the electrons on an atom etermines how it bonds withother atoms
what are electron chells
rings around the nucleus that deteremine the energy levels of the electrons (bigger the ring bigger the energy vice versa) Represented by the symbol n
how many electrons can the shells hold
1- 2 electrons 2.8electrons
What are Valence electrons
they are electrons on an atoms that will participate in bonding with another atom (outermost electron shell)
what causes for an atom to be more chemically stable
when they fill up their outermost electron shell or valance shell with electrons
how are rows organizned in the periodic table
by electron shells
what is a chemical bond
when atoms from a lastong attraction to eachother
what is a chemical reaction
wgen atoms or molecules interact with each other and change their arrangemnt or configuration of chemical bonds
what are reactants
substances that are presetn at the start of a reaction
what are products
substances that are formed during a reaction and are present at the end
what is the most common type of chemical bond
a covalent bond
how do covalen bonds from
betwhent wo atoms share pairs of electrons
what happens when two atoms form a covalent bond
they become chemically stable by fillinf each other valence orbitals (rings)
which bond is the most stable
Covalent bonds
what is a molecule
when two or more atoms form a covalent bond
what is a nonpolar covalent bond
when there is a similar amount of electronegatcity an the electrons are equally shared
what does electronegativity do
it attracts a bonding pair of electrons
what is a polar covalent bond
its when one atom in the bond har much higher electro negativty thant eh toher thent he electrons are not equally shared
what is an ion
when an atom picks up or loses electrons to achieve a stbale electron number when it fully gaines orloses the electron then it causes an electrical charge
What symbol is showns next to the leement symbol
it is either a + or - sign and it shows how many lectrons or lost or gained
what is a cation
when an atom LOSES one or more electrons becomes positive it gets called a cation
what is an anion
when an atom GAINS one ore more electrons it becomes negative and called an anion
when are inoic bonds formed
when oppositely charged ions are attracted to each other and the sharing of electronis more in favor of the electronegatice atom
what happens when inic bonds frome in grater numbers in the same area
they form altnernating sheets whic form solid cryslaline material
what is a hydrogen bond
it is a weak attraction between two different molecules with oppostie partial charges
how does a hydongen bond form
when there is a positively charged hydrogen atom on one molecule and a negative electronegative atom on another molecule
what is cohesion
when molecules stick to each other more than in other liquids
what is adhesion
when water dropends cling on other surfaces
what is high thermal capacity
when the product can hold alot of energy; it takes a lot of energy to get the molefulesto come apart
what is hydrophilic
water loving, it can interact or bond with water molecules on the molcular level
why are polar molecules attrated to water
because of their charges )opposites attract
what is hydrophobic
water fearing - when it does not interact or bond with water molecules
what type of molecules are hydrophobic
nonpolar molecules or neutral substances
what is a solution
it is a solution uniform liquid mixture of two or more substances
what is solvent
when a substance in a solutons dissolves other sibstances
what is a solute
a substance that dissolves in a solution
what is a pH
it is the measure of acidity or basicity of a solution
what is acid (Acidity)
it is a substance that realaeases hydrogens and choride ions
wht does acid do to a substance
it increases the concetration ofof hydogen ions and will make a chemical come apart into indivdual atoms or ions
what is base
a substance that increses the concetratio nof hydroxide ions in a solutions is one of two basic ways; bonding with hydrogen ions or realeasing hydroxide ions
what does a base subtance do
it brings together hydrogen atoms , ions or other atoms to make a chemical
why can acids and bases neutralize each other
because they have the oppostife effect on the concetration of hydrogens in a sollution
what is the pH scale used for
to show the relative aitity or basicity of s sulution
from what numbers does the scale end and start
from 0 to 14
where in the scale would it be considered neutral
7
what is the scale based on
the ratio of hydrogen and hydroxides
from which number does the acidic side start and end
starts at 7 and ends at 0
from which number does the basic side start and end
it starts at 7 and ends at 14
which side has a higher amounf of hydrogens (H+) comapted to neutral
the acidic side
which side has a lower amount of hydrogens (H+) compared to neutral
the basic side
the pH scale is logarithmic which means
that the difference from one whole number to the next is a 10x concentration shift of hydrongens (H+)
what are buffers
theya re solutions that help minimize changes in pH by accpeting or donating Hydronges (H+) in a solution
true or false: can buffers act as a base or an acid depending on the sutiation
True
what is teh bicarbonate blood buffer system
a BUFFER
how does the bicarbonate blood buffer system work
an enzyme converts carbon dioxide and water into carbonic acid(acid) . tehn some of the carbonic acid dissociates into bicarbonate and hydrogen ions (base). it creates both an acid and a base to neutralize each other and other acids and bases that enter the blood stream