Reduction of Aldehydes and Ketones , Nucleophilic addition
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
Aldehydes and ketones can be reduced to form what?
Primary and secondary alcohols.
Aldehydes are reduced to form…
Primary alcohols
Ketones are reduced to form…
Secondary alcohols.
What reducing agent is used when reducing aldehydes / ketones into alcohols?
Sodium tetrahydridoborate(III), NaBH4
Lithum tetrahydridoaluminate(III) LiAlH4.
NaBH4 is preferred as it is safer and can be used in aqueous conditions whereas LiAlH4 can form explosive gases.
What symbol is used to represent reducing agents?
[H]
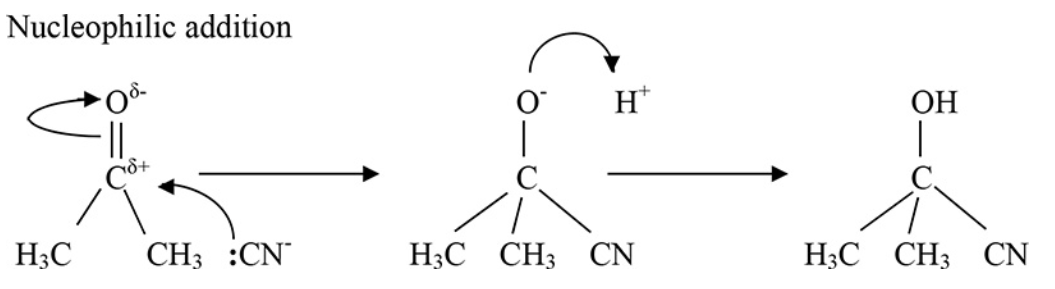
State one distinct characteristic about the C=O found in aldehydes and ketones.
The carbonyl group C∂+= O∂- is polar.
The carbonyl group in aldehydes and ketones C∂+= O∂- is polar. What does this mean?
The electron deficient carbon can be attacked by nucleophiles (a species that contains an electron pair available for bonding) such as the :NC- (cyanide) ion.
Draw the mechanism showing the nucleophilic addition of the CN- ion to a carbonyl.
Aldehydes and ketones readily undergo addition by hydrogen cyanide (HCN) to form hydroxynitriles.
HCN is created in situ from a mixture of aqueous potassium or sodium cyanide and sulfuric acid.