NCEA Level 2 Chemistry - Bonding, Structures, Properties and Energy Changes
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
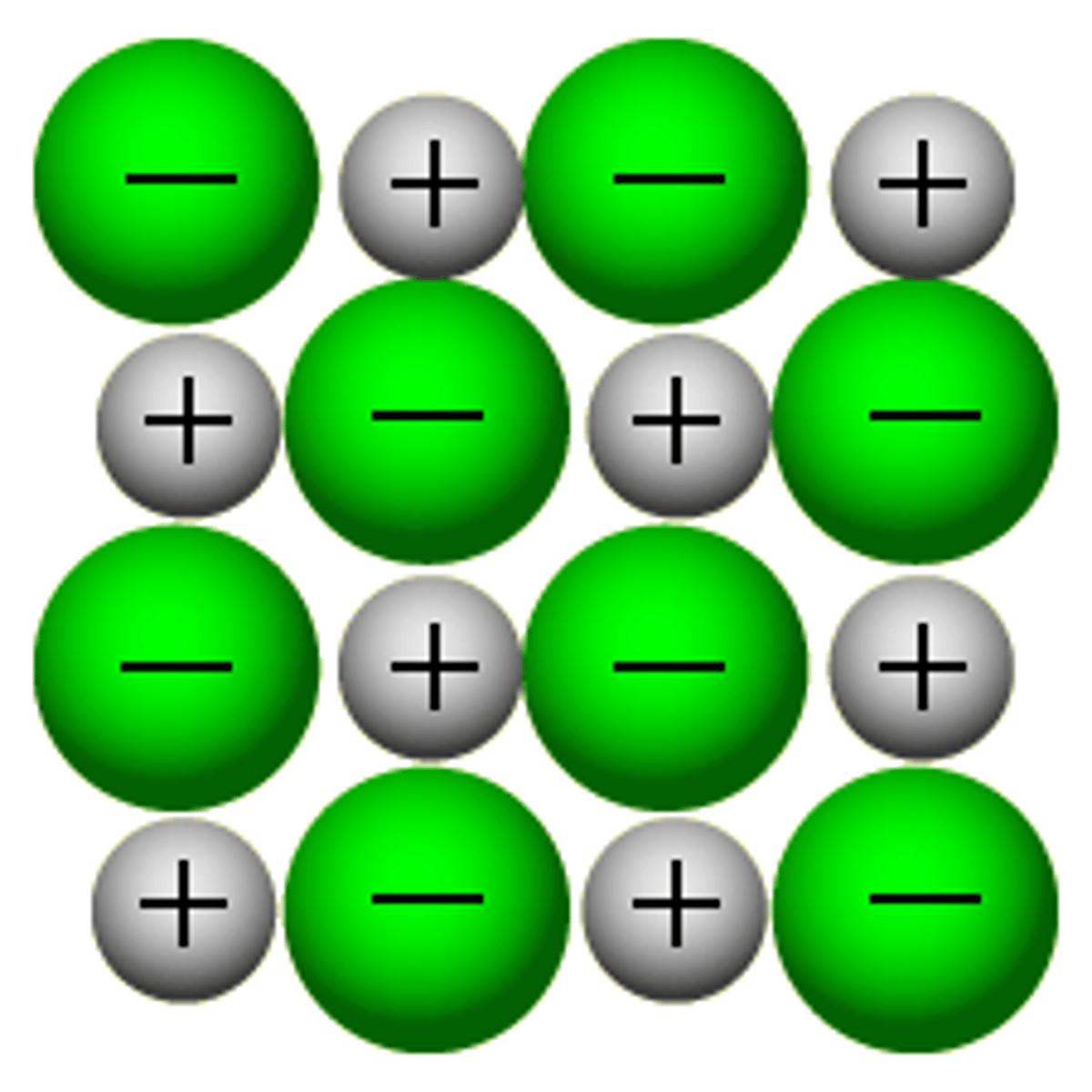
Ionic Solids
Particles: cations and anions
Attractive Forces: very strong electrostatic attractions between ions
Bond type: ionic bond
Properties of ionic solids
- Brittle
- High melting point
- Conductivity (electrical)
- Solubility
Why are ionic solids brittle?
Because when struck, the ions of the same charge line up, causing repulsion and a split in this plane occurs
Why do ionic solids have a high melting point?
It takes a lot of energy to overcome the strong electrostatic forces and break ionic bonds between ions
Conductivity of ionic solids
Ionic solids only conduct in molten (l) or aqueous (aq) form, not as a solid.
To be able to carry an electrical current a substance must:
1. Contain charged particles (ions, electrons)
2. Be mobile (free to move)
In the solid state, the charged ions are held in a rigid 3D lattice structure and are not mobile and therefore do not conduct. However, in liquid (molten) and aqueous state there are charged particles (ions) that are mobile and so ionic solids do conduct and are able to carry a current
Solubility of ionic solids
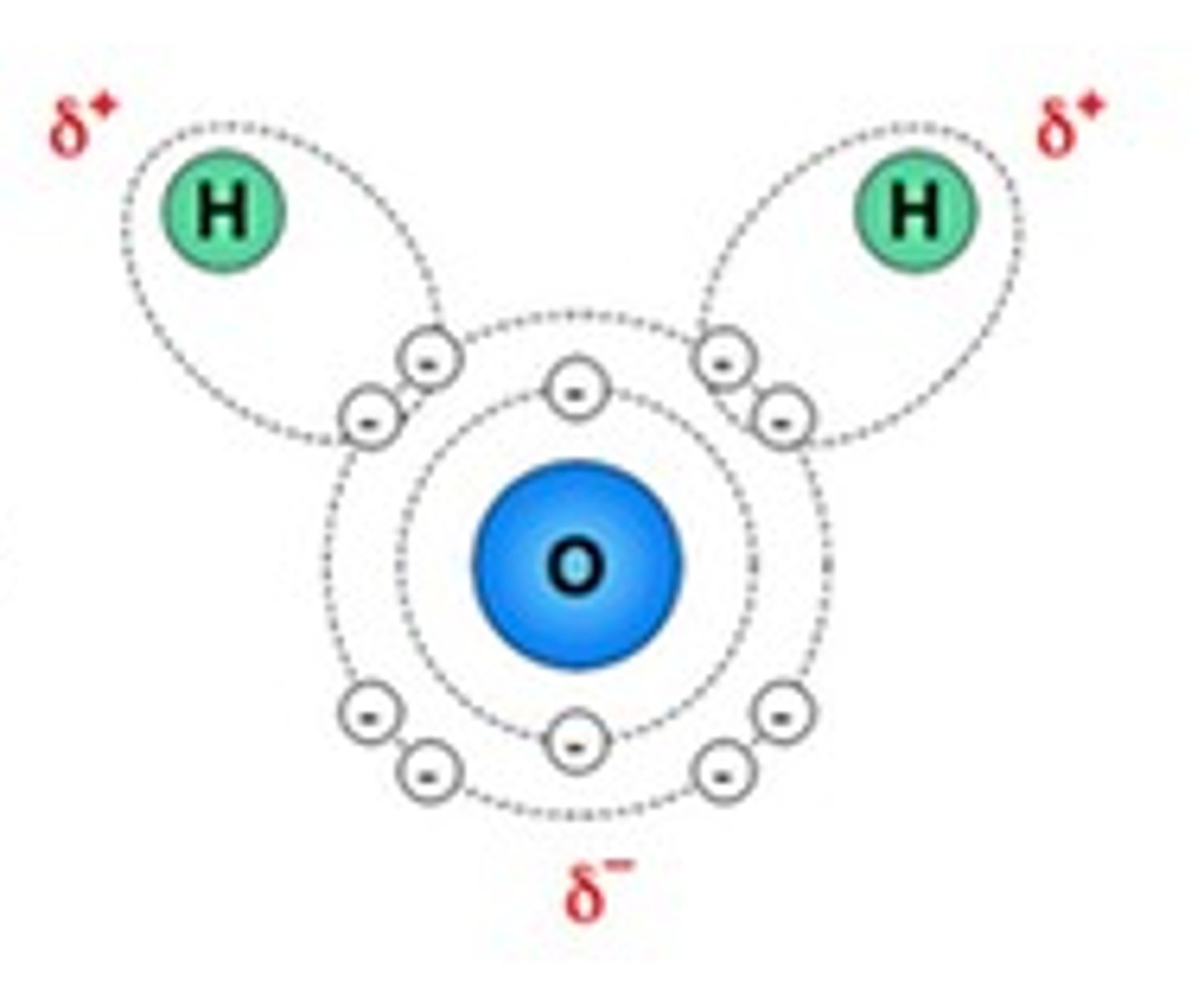
Ionic solids dissolve in water because the force of attraction between the ions can be overcome by the force of attraction between the ions and water molecules. The lone electron pairs on the O atom make this end of the molecule more negative. In addition there is uneven sharing of the bonding electrons in the two H-O bonds of water. Oxygen more strongly attracts the bonding electrons (oxygen is more electronegative) than hydrogen. The hydrogen atoms are therefore less electronegative. The positive ions are therefore attracted to the O of water (delta negative) and the negative ions are attracted to the H atoms (delta positive)
Metallic substances
Particles: atoms in a sea of delocalised valence electrons
Attractive forces: strong electrostatic attractions between atoms and mobile electrons
Bond type: metallic bonds
Properties of metallic substances
- Malleable/ductile
- High melting point
- Conductivity (electrical)
- Conductivity (heat)
Why are metallic substances malleable/ductile?
Malleable means capable of being pressed or hammered into shape. In a metal the positive nuclei of atoms are attracted to the valence electrons of adjacent atoms, in all directions. This creates strong electrostatic attractions that are non-directional, therefore atoms can move past one another without disrupting the metallic bonds, therefore metals are malleable and ductile (able to be drawn into a wire)
Why do metallic substances have a high melting point?
A large amount of energy is required to overcome and break the strong metallic bonds
Electrical conductivity of metallic substances
Metallic substances are able to conduct when solid or liquid. Delocalised valence electrons are free to move in both solid and liquid state
Heat conductivity of metallic substances
Delocalised electrons mean they are free to vibrate, bump into each other passing the heat energy along the metal to transfer heat energy from one end to another
Covalent Molecular Solids (Polar)
Particles: polar molecules
Attractive forces and bond types: electrostatic attraction between dipoles of intermediate strength
Covalent Molecular Solids (Non-Polar)
Particles: non-polar molecules
Attractive forces and bond types: weak intermolecular bonds between molecules. Strong covalent bonds within molecules
Properties of covalent molecular solids
- Low melting and boiling points
- Do not conduct electricity
- Solubility
Why do covalent molecular solids have low melting and boiling points?
Only a small amount of energy is needed to overcome/break the weak intermolecular bonds (only the bonds between the molecules, the covalent bonds inside the molecules do not break)
Why don't covalent molecular solids conduct electricity?
They have no free electrons or ions to carry a current
Solubility of polar covalent molecular solids
Covalent molecular solids made up of polar molecules have an uneven distribution of charge across the molecule. Most are soluble in polar solvents, especially when there are less than 4 carbon atoms in the backbone of the molecule. Attractive forces exist between the delta positive and delta negative atoms/parts of the polar molecule and the delta positive and delta negative atoms/parts of the solvent
Solubility of non-polar covalent molecular solids
Non-polar substances are not soluble in polar solvents (water) because water molecules are more strongly attracted to other water molecules than to the non-polar substance. However, they may dissolve in non-polar solvents
Linear Covalent Network Solids
Particles: long chain molecules (polymers)
Attractive forces/bond type: strong covalent bonds
Properties:
- Elastic to hard
- Low to medium melting points
2D Covalent Network Solids
Particles: atoms
Bond type: each atom is covalently bonded to 3 others in a triangular shape in a 2D arrangement, leaving one electron that is delocalised and free to move
Attractive forces: strong covalent bonds between atoms within layers. Weaker forces between layers
Properties of 2D Covalent Network Solids
- High melting point because a large amount of energy is required to overcome and break the strong covalent bonds between atoms within layers
- Soft and used as a lubricant because the weak attractions between the layers of atoms are easily broken. Therefore they are soft and slippery and layers can slide over each other, making it useful as a lubricant
- Because each atom is covalently bonded to 3 others, leaving one electron that is delocalised and free to move, 2D covalent network solids can conduct electricity
3D Covalent Network Solids
Particles: atoms
Bond type: each atom is covalently bonded to 4 other atoms in a tetrahedral arrangement, producing an extended 3D array
Attractive forces: very strong covalent bonds between atoms
Properties of 3D Covalent Network Solids
- Strong with a high melting point because the very strong covalent bonds between atoms require a very large amount of energy to be overcome and break
- Does not conduct because there are no free electrons or ions to carry a current
- Insoluble
Describing the shape of a molecule
1. Name and formula of the molecule
2. Draw the Lewis structure
3. State the number of areas of electron density around the central atom
4. State that the areas of electron density take up positions of maximum repulsion creating a particular arrangement and bond angle
5. State the number of single bonds/multiple bonds versus the number of non-bonding pairs
6. State that the bonded electron positions can be seen and while the non-bonded electron pair positions contribute to the shape, they cannot be seen in the final shape therefore the shape of the molecule is...
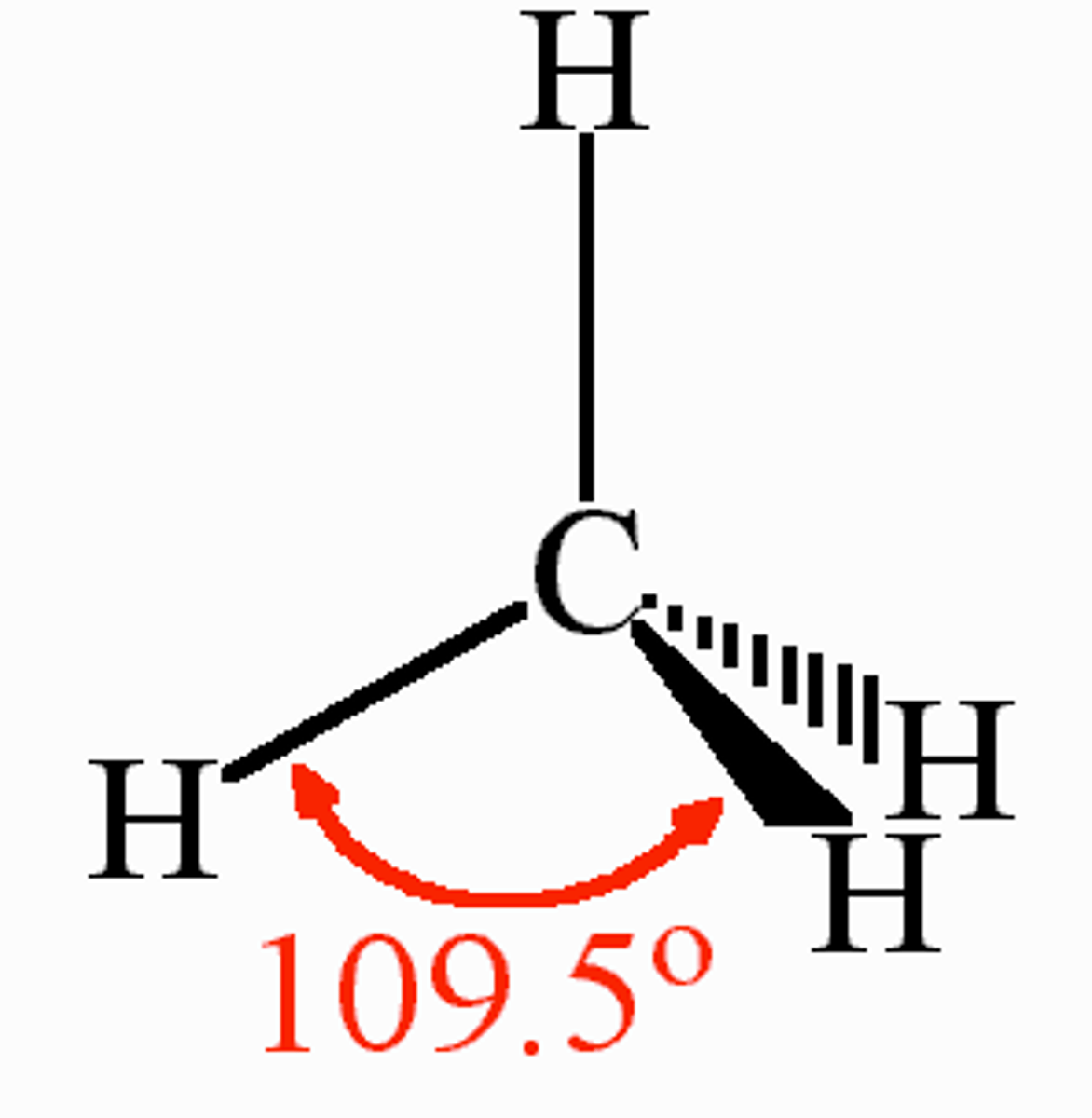
Tetrahedral shape
Regions of electron density: 4
Arrangement: tetrahedral
Occupied sites: 4
Bond angle: 109.5°
Trigonal pyramid shape
Regions of electron density: 4
Arrangement: tetrahedral
Occupied sites: 3
Bond angle: 109.5°
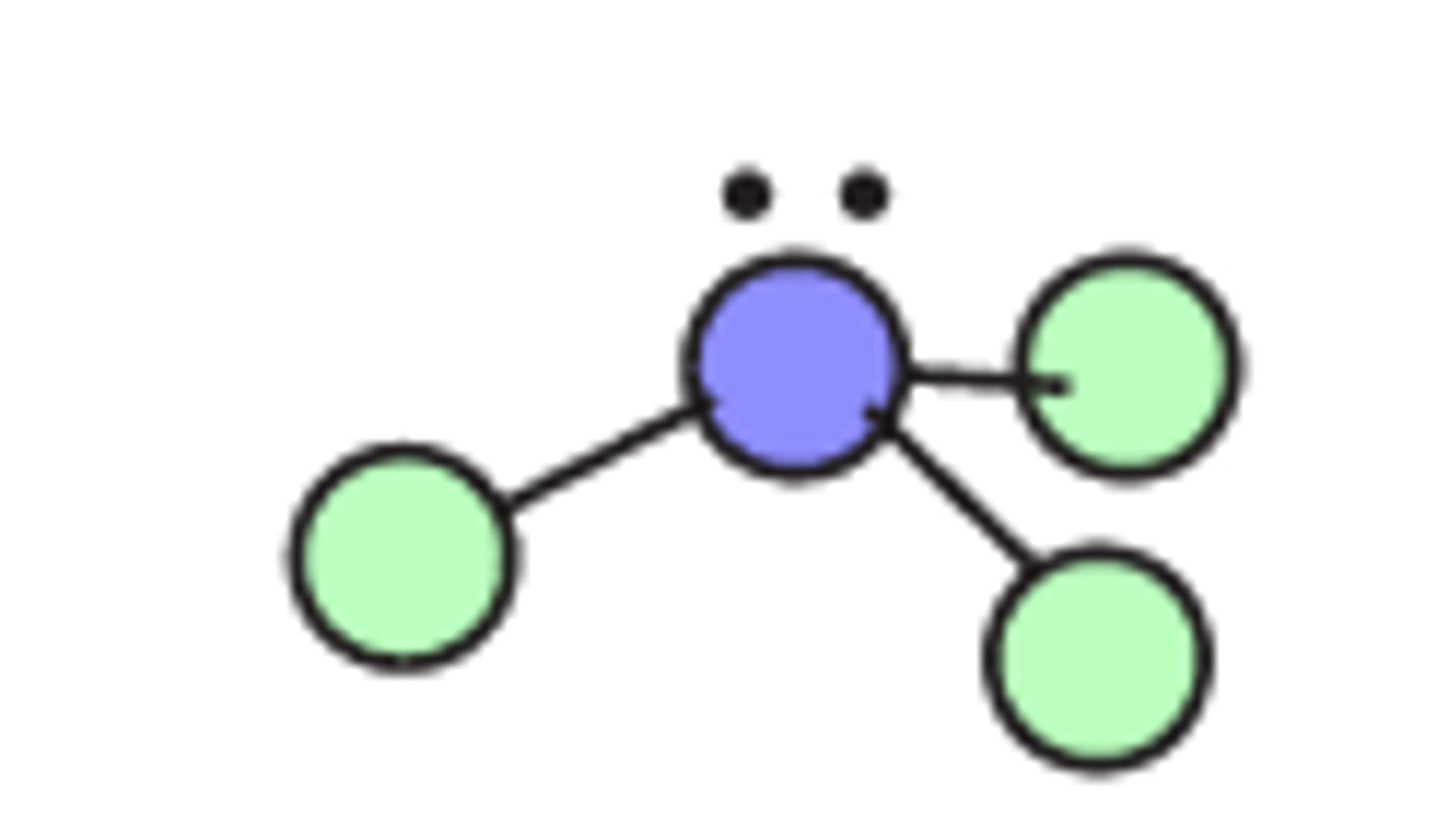
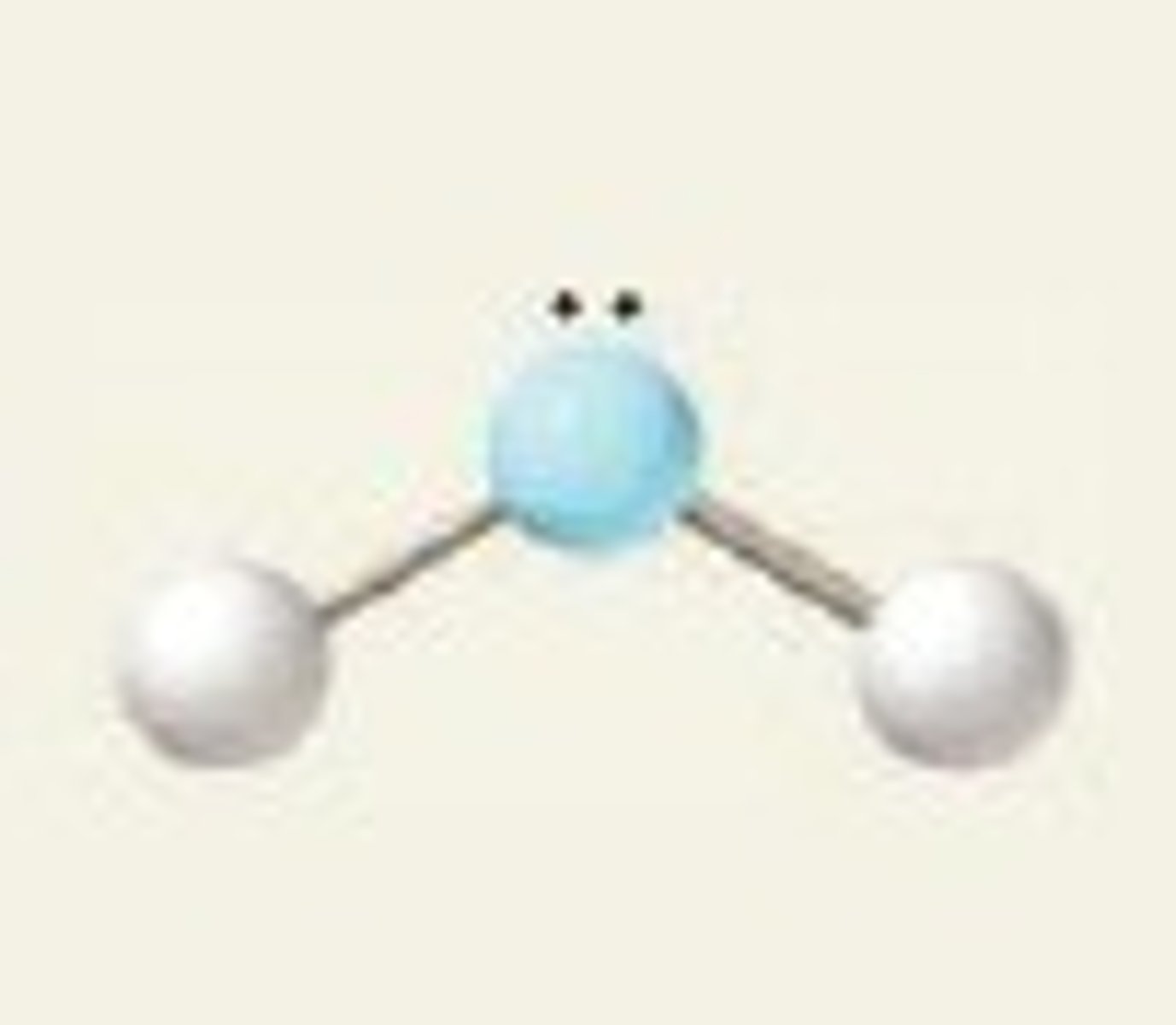
Bent shape
Regions of electron density: 4
Arrangement: tetrahedral
Occupied sites: 2
Bond angle: 109.5°
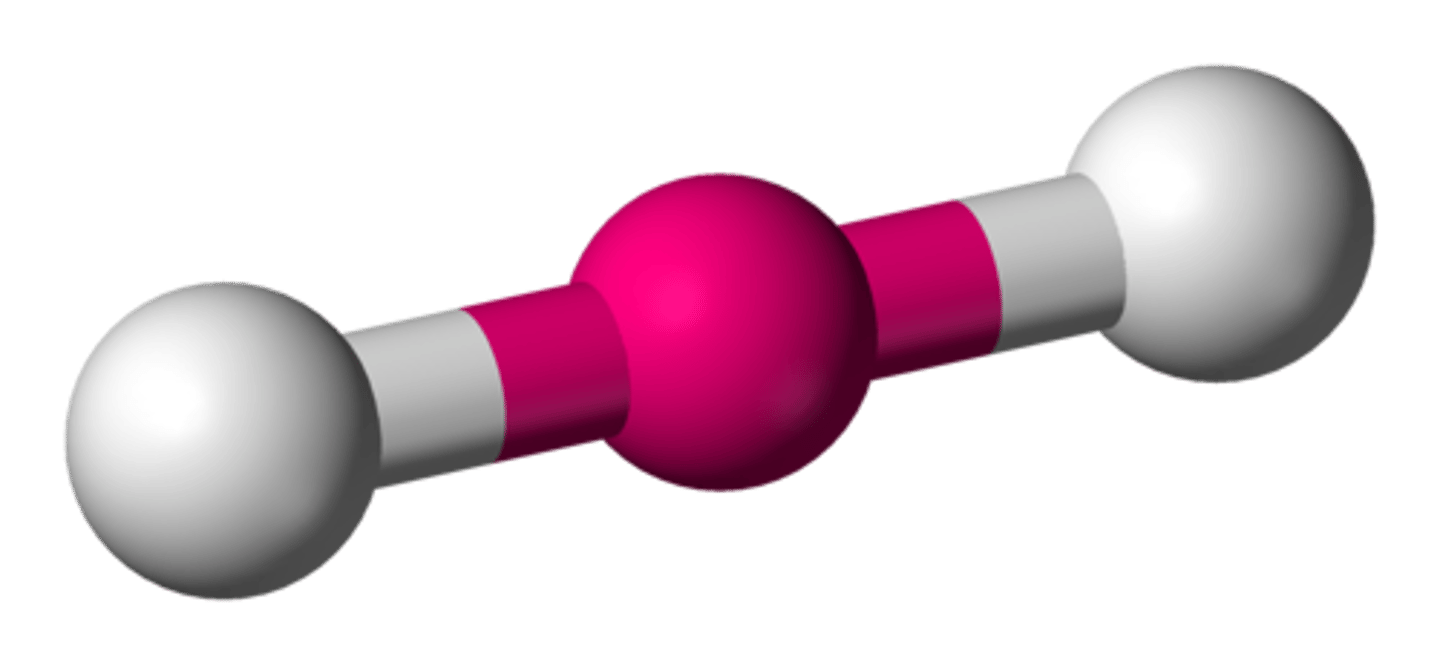
Linear shape (4 regions of electron density)
Regions of electron density: 4
Arrangement: tetrahedral
Occupied sites: 1
Bond angle: 180°
e.g. H-Cl
Linear shape (2 regions of electron density)
Regions of electron density: 2
Arrangement: linear
Occupied sites: 2
Bond angle: 180°
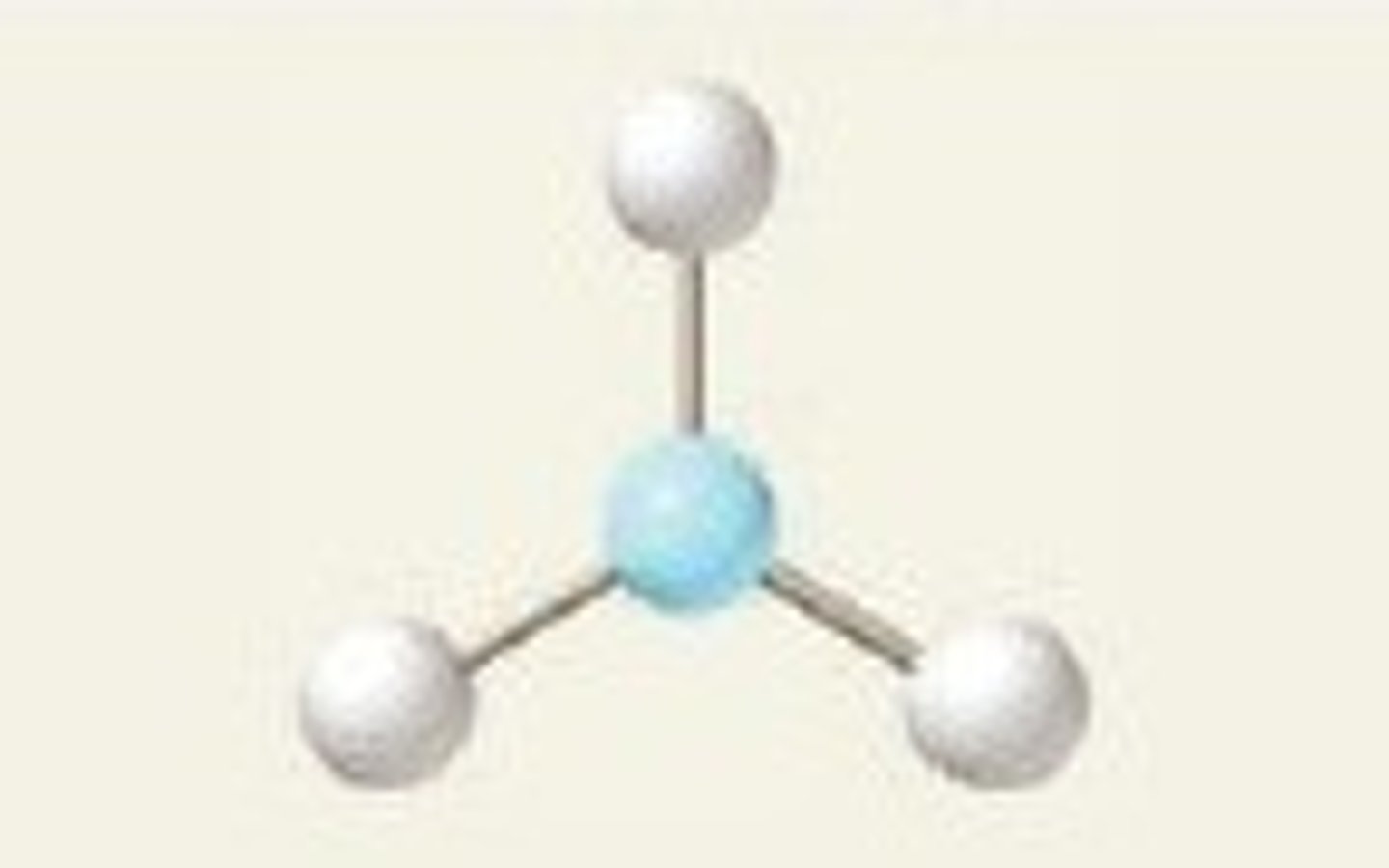
Triangular (trigonal planar) shape
Regions of electron density: 3
Arrangement: triangular
Occupied sites: 3
Bond angle: 120°
How to describe polarity of a molecule
1. Describe the shape of the molecule
2. Say that each bond is polar due to differences in the electronegativity between the atoms, creating dipoles (uneven charge distribution across the bond)
3. If the molecule is symmetric, the bond dipoles cancel each other out and the molecule is non-polar. If there is an asymmetric arrangement of atoms around the central atom, the bond dipoles do not cancel and the molecule is polar
Chemical reactions
Chemical reactions are always accompanied by a change in energy. When chemical reactions occur, new chemical bonds are formed between reactants and existing chemical bonds have to be broken. The energy required for this to occur is called activation energy. To break chemical bonds energy needs to be provided, and when chemical bonds are formed energy is released
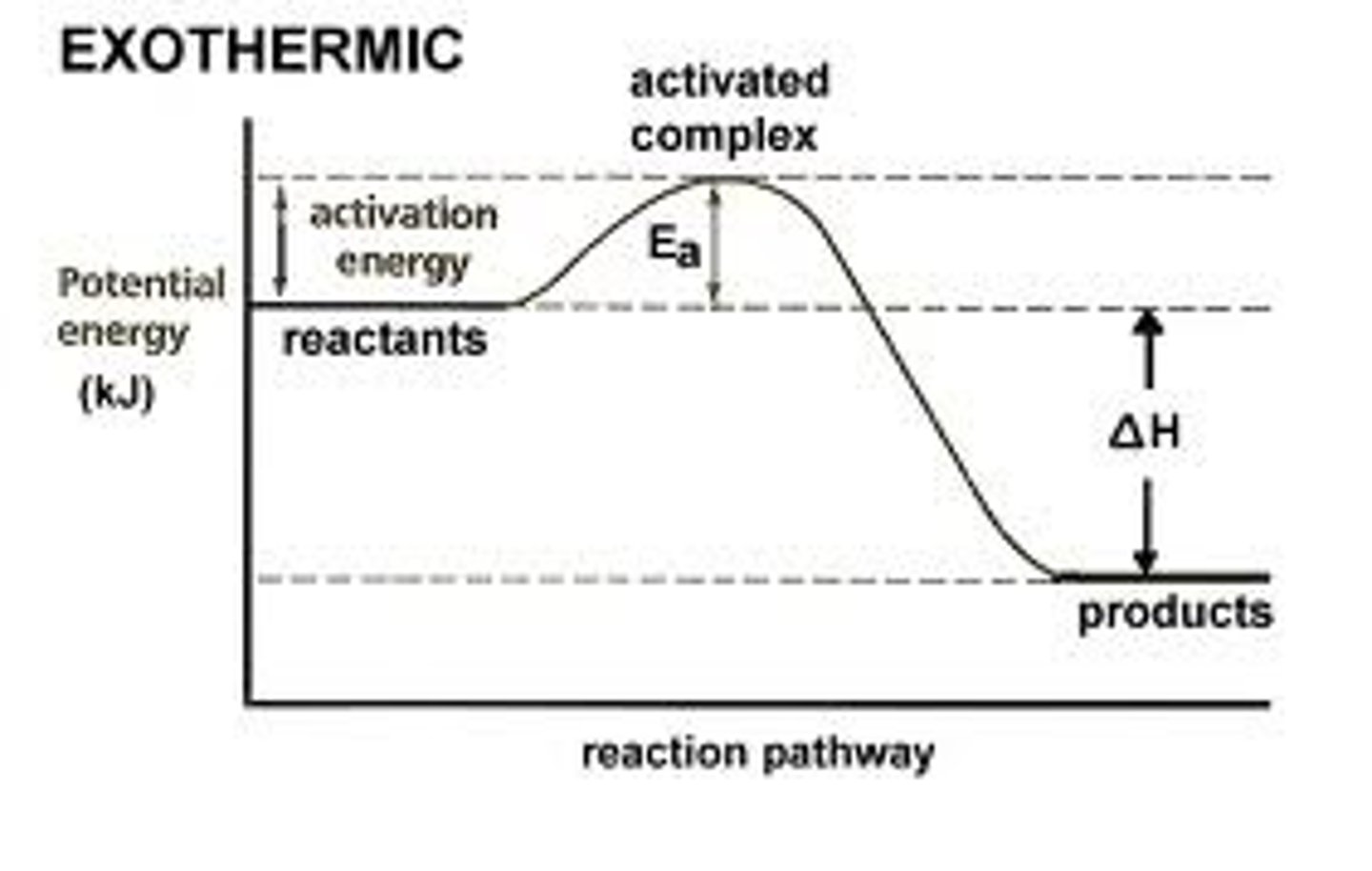
Exothermic reactions
If in the formation of new bonds more energy is released than is required to break the existing bonds, heat is given out. This is called an exothermic reaction. Exothermic reactions are recognised by the temperature of the surroundings increasing. The reactants will have more energy than the products. ΔH will always be negative
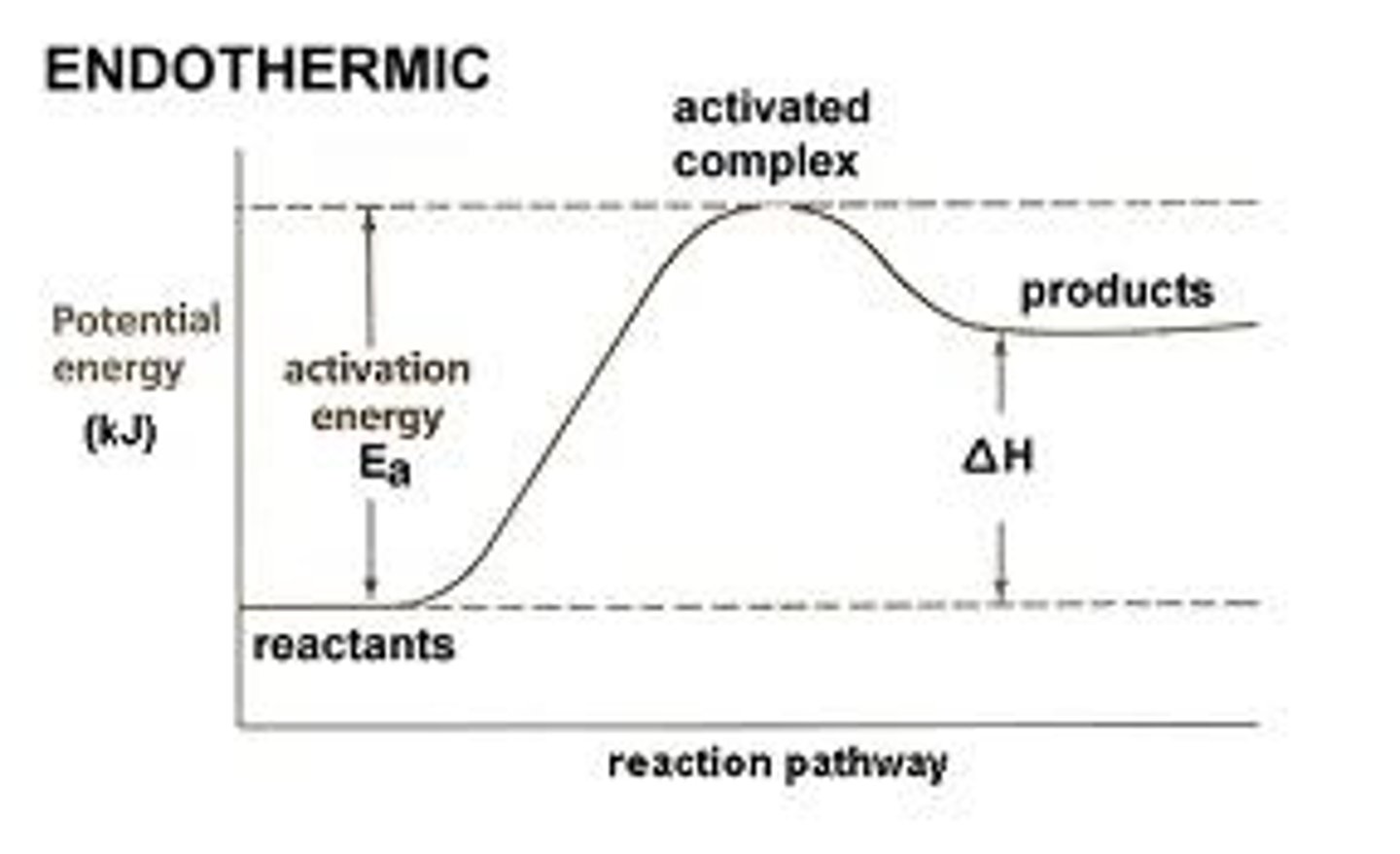
Endothermic reactions
If more energy is required to break existing bonds than is released when new bonds are formed, heat is absorbed. This is an endothermic reaction. Endothermic reactions are recognised by the temperature of the surroundings decreasing. The reactants will have less energy than the products. ΔH will always be positive