Chem entropy/spontaneity
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Entropy (S) def
A measure of the dispersal or distribution of matter/energy
When moving to more disorder (s→g), does entropy increase or decrease?
Increase (positive change)
Does solid, gas, or liquid have the most entropy?
Gas
When explaining changes in entropy, what 2 things should you talk about?
Changes in state (s, l, aq, g)
Number of moles (of stuff in each state)
What’s the 2nd law of thermodynamics?
Spontaneous processes always occur with an increase in the entropy of the universe.
Absolute entropy def
Entropy under standard temperature and pressure conditions
What’s the equation for calculating ΔSƟ using standard entropy values?
ΔS⊝ = ΣS⊝(products) - ΣS⊝(reactants)
Gibbs energy (G) def
The energy produced by a system that can be used to do work - the energy left over after the heat energy (enthalpy) is used to disperse particles (entropy)
What does it mean if the ΔG is negative?
The reaction is spontaneous
What’s the equation for calculating ΔG using Gibbs energy of formation values?
ΔG⊝ = ΣΔGf⊝(products) - ΣΔGf⊝(reactants)
Using ΔG (system) = ΔH (system) - TΔS (system), what determines the sign of ΔG when the temperature is low?
Sign of the enthalpy change - T is basically zero so entropy change doesn’t matter bc that whole thing is zero. So, if exothermic (-H) then G is negative/spontaneous and if endothermic (+H) then G is positive/not spontaneous
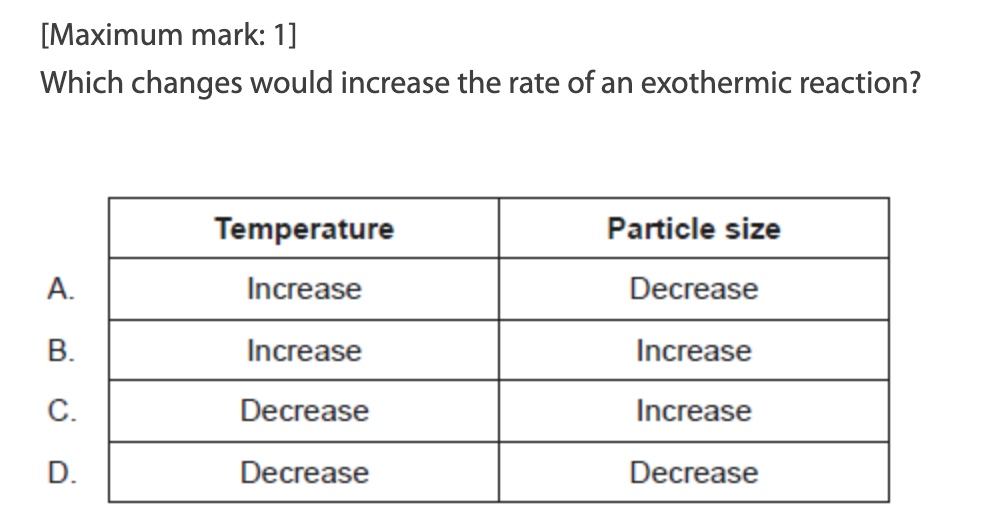
A
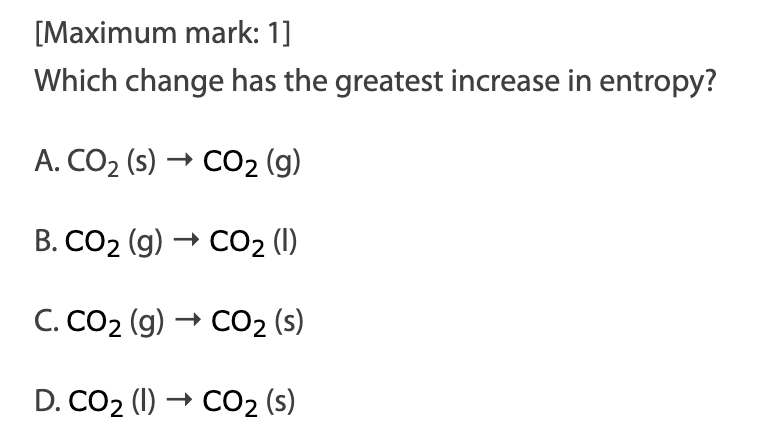
A