Chemistry ahhhhhhhhhhhhh
1/16
Earn XP
Description and Tags
lalalalalala
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
What determines the identity of the atom
Proton # = Atomic #
Identity of an atom
element
What determines the isotope of the element
neutron
Isotope
Variants of an element that have the same number of protons but different numbers of neutrons.
Ion
a charged particle formed when an atom gains or loses electrons.
What determines the charge of the atom
the number of electrons (charge = pprotons - electronsro
number at the top of a element or to the bottom left
Atomic #
number below an element or to the top left
Mass #
number to the top left
the charge
Charge, mass, and location of proton
1amu, neucleus, +/ cation
Charge, mass, and location of neutron
1amu, nucleus, neutral
Charge, mass, and location of electrons
negative, 0.000548 amu, electron cloud
Average atomic mass formula
(%1 x mass1) + (%2 x mass2) / 100
What differentiates isotopes of the same element
Isotopes of the same element differ in the number of neutrons in their nuclei, leading to variations in their atomic masses.
Alpha decay
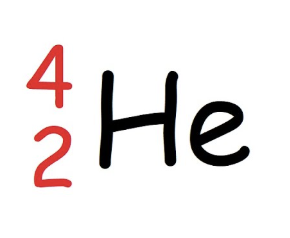
Beta decay
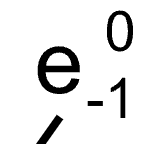
Gamma ray decay