Chem Chapters 8,9,11 (Study Guide for Exam 4)
1/66
Earn XP
Description and Tags
Chapter 8: Metabolism Chapter 9: Cellular Respiration Chapter 11: Cell Communication
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
Local distancing in Cell
Cells in a multicellular organism communicate via signaling molecules
▪ In local signaling, animal cells may communicate by direct contact through cell junctions.
Local Signaling
Electrical signal triggers release of neurotransmitter.
Neurotransmitter diffuses across synapse.
Long Distant Signaling
Hormone travels in bloodstream.
Target cell specifically binds hormone.
Three Stages of Cell Signaling
Reception - Transduction - Response
Signal Reception
The binding between a signal molecule (ligand) and
receptor is highly specific
Signal Transduction
Relay molecules transmit the signal, ultimately activating an enzyme.
Cell Response
The enzyme breaks down glycogen, releasing glucose that fuels the area.
G-Protein Coupled Receptors structure and function
Structure and Function:
GPCRs: These receptors span the cell membrane and have seven transmembrane alpha helices. They bind signaling molecules outside the cell and interact with G proteins inside the cell.
G Proteins: These proteins bind guanosine triphosphate (GTP) and guanosine diphosphate (GDP). They act as molecular switches, being active when bound to GTP and inactive when bound to GDP.
G-protein-coupled receptors (GPCRs) are the largest and most diverse group of membrane receptors in eukaryotes. These cell surface receptors act like an inbox for messages in the form of light energy, peptides, lipids, sugars, and proteins.
Receptor Tyrosine Kinases structure and function
They have a multitude of Tyrosines attached to one end.
Why these Tyrosines are activated with Phosphate it uses 6 ATP and creates 6 ADP
When fully activated receptors, they can activate rely proteins as they attach to them.
Process of cell signaling using an intracellular receptor.
Cell signaling using intracellular receptors involves a signaling molecule, often a hormone, entering the cell and binding to a receptor within the cytoplasm or nucleus, triggering a change in gene expression
Phosphorylation Cascade
A phosphorylation cascade is a sequence of signaling pathway events where one enzyme phosphorylates another, causing a chain reaction leading to the phosphorylation of thousands of proteins. This can be seen in signal transduction of hormone messages.
Type of molecule Kinase is and what does it do?
A kinase is a type of enzyme (a protein that speeds up chemical reactions) that transfers phosphate groups from high-energy molecules, like ATP, to other molecules, such as proteins or lipids, a process called phosphorylation, which can alter the activity or function of those molecules.
The role of Kinases in signaling pathways.
Signaling kinases often induce a cascade that results in the phosphorylation of several proteins or molecules within the cell. These cascades alter cellular function. Thus, these proteins 'signal' changes in cells.
Type of molecule is a phosphatases and what it does.
Phosphatases are hydrolases that catalyze the removal of a phosphate from a phosphoric acid monoester in a reaction involving nucleophilic attack on the phosphorus atom in the presence of water
Role of phosphatases in signaling pathways.
Phosphatases play a vital role in cellular signalling by controlling both the network dynamics and spatial localisation of phosphoproteins
Steps of different types of G-protein signaling pathways.
Structure and Function:
GPCRs: These receptors span the cell membrane and have seven transmembrane alpha helices. They bind signaling molecules outside the cell and interact with G proteins inside the cell.
G Proteins: These proteins bind guanosine triphosphate (GTP) and guanosine diphosphate (GDP). They act as molecular switches, being active when bound to GTP and inactive when bound to GDP.
Cellular responses to cell signaling.
Signals (a.k.a. ligands) and receptors come in many varieties, and binding can trigger a wide range of signal relay cascades inside the cell, from short and simple to long and complex.
At the molecular level, we can see changes such as an increase in the transcription of certain genes or the activity of particular enzymes.
At the macroscopic level, we may be able to see changes in the outward behavior or appearance of the cell, such as cell growth or cell death, that are caused by the molecular changes.
Signal Amplification
The amplification of signals can be defined as an increase in the intensity of a signal through networks of intracellular reactions.
Signal Specificity
Signal specificity refers to the ability of cells to respond uniquely to specific signals while ignoring others. This ensures that each signal triggers the appropriate response within the cell, allowing for precise communication and coordination of cellular functions.
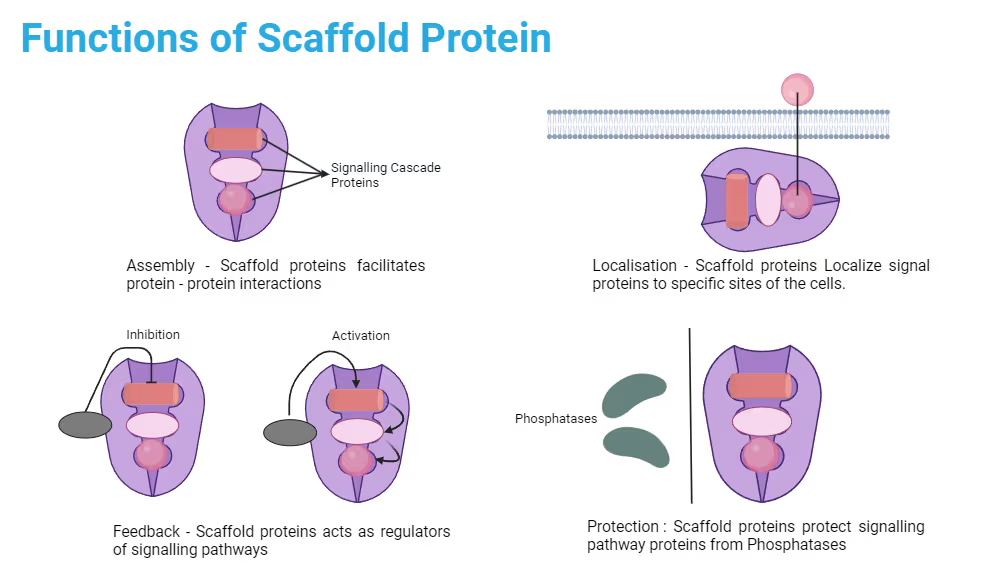
Scaffold Proteins
Proteins that simultaneously bind two or more other proteins, and organize binding partners into a functional unit to enhance signaling efficiency and fidelity.
Apoptosis and its biological significance.
Apoptosis is a biological process which occurs in all multicellular organisms including plants and animals. It removes the cells from the organisms that should no longer be a part of the organism. This process plays a major role in the development of humans and in developing and maintaining a healthy immune system.
Metabolism
Metabolism refers to the whole sum of reactions that occur throughout the body within each cell and that provide the body with energy.
Catabolic pathways
This type of pathway releases energy and is used to break down large molecules into smaller ones (degradation).
Anabolic Pathways
This type of pathway requires energy and is used to build up large molecules from smaller ones (Biosynthesis).
Forms of energy and conversion process
Thermal Energy, Mechanical Energy, Solar Energy, Chemical Energy, Kinetic Energy, Nuclear Energy.
1st Law of Thermodynamics
Energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be created nor destroyed, under any circumstances.
2nd Law of Thermodynamics
The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time.
The second law also states that the changes in the entropy in the universe can never be negative.
Gibbs Free Energy (The Equation)
Delta G = DeltaH (Change in Enthalpy) - TDeltaS (Temperature in Kelvin + DeltaS Change in Entropy.

Entropy
A thermodynamic quantity representing the unavailability of a system's thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system.
Lack of order or predictability; gradual decline into disorder.
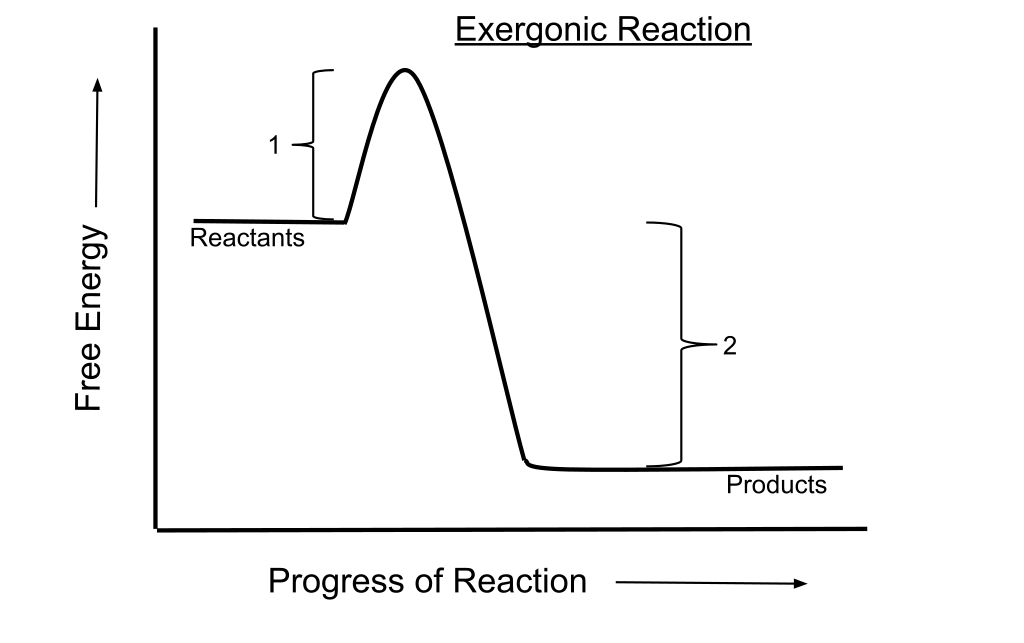
Exergonic Reactions
Exergonic reactions are chemical reactions that release energy in the form of heat. It is a spontaneous reaction.
Endergonic Reactions
Endergonic reactions are non-spontaneous, meaning that energy must be added before they can proceed. You can think of endergonic reactions as storing some of the added energy in the higher-energy products they form .
Equilibrium in chemical reactions
Condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and products occurs.
Equilibrium in biology?
Living cells are intrinsically non-equilibrium systems, driven by energy-consuming processes like molecular motors and enzymatic reactions.
Cells require a constant input of energy to maintain this dynamic steady state and prevent them from reaching equilibrium, which would lead to death.
Open Systems
An open system is one in which energy can be transferred between the system and its surroundings.
Closed Systems
A closed system is one that cannot transfer energy to its surroundings.
ATP details of its hydrolysis and synthesis
ATP hydrolysis is the process where ATP breaks down into ADP and inorganic phosphate (Pi), releasing energy that cells use to power various processes like muscle contraction and chemical reactions.
Primarily through cellular respiration, specifically via oxidative phosphorylation in the mitochondria, where a proton gradient drives the enzyme ATP synthase to convert ADP and inorganic phosphate into ATP.
Importance of ATP
The cell's energy currency
Types of Cellular work fueled by ATP
ATP fuels cellular work in three main ways:
Chemical (synthesis of molecules)
Mechanical (muscle contraction)
Transport (moving molecules across membranes)
Energy Profile of Exergonic reaction
A chemical reaction where the change in the free energy is negative.
Enzymes (Structure and Function)
Enzymes are proteins that act upon substrate molecules and decrease the activation energy necessary for a chemical reaction to occur by stabilizing the transition state. This stabilization speeds up reaction rates and makes them happen at physiologically significant rates.
Events in Active Site of Enzyme that can decrease energy of activation.
Bringing reactants together, positioning them correctly for reaction, and potentially participating directly in the reaction through catalytic residues, thereby facilitating faster reactions.
The events of an enzymatic reaction.
The enzyme binding to a specific substrate at its active site, forming an enzyme-substrate complex, which then undergoes a chemical transformation into a product, which is subsequently released, leaving the enzyme free to catalyze further reactions.
How is enzyme activity affected by different temperatures?
Enzyme activity is affected by temperature; generally, enzyme activity increases with temperature until an optimal point, after which it decreases due to enzyme denaturation.
How is enzyme activity affected by different pH?
Enzyme activity is significantly affected by pH, with each enzyme having an optimal pH range for maximum activity; deviations from this range can lead to reduced activity or even denaturation.
How is enzyme activity affected by different cofactors?
Cofactors, including ions and coenzymes, are essential for enzyme activity, as they bind to specific enzymes, facilitating optimal conformation and function, and thereby increasing the specificity of enzyme-substrate interactions.
How is enzyme activity affected by different substrate concentrations?
Enzyme activity initially increases with rising substrate concentration, but eventually plateaus as the enzyme becomes saturated, reaching a maximum reaction rate (Vmax).
Metabolic Pathway
A series of biochemical reaction within a cell that convert molecules or substrates into other materials, facilitated by enzymes, and can be either anabolic (building up) or catabolic (breaking down).
Inhibition Allosteric Regulation
Allosteric inhibition is a type of enzyme regulation where a regulatory molecule (inhibitor) binds to a site (allosteric site) other than the active site, causing a conformational change that reduces the enzyme's activity or prevents substrate binding.
Feedback Control
Feedback control systems, also known as closed-loop control systems, use the output of a system to adjust its inputs, aiming to maintain a desired state or output by minimizing the difference between the desired and actual values.
How do photosynthesis and cellular respiration interplay?
Photosynthesis and cellular respiration are interconnected processes; photosynthesis uses carbon dioxide and water to produce glucose and oxygen, and cellular respiration uses glucose and oxygen to produce carbon dioxide and water, forming a cyclical relationship.
Equation of cellular respiration.
C6H12O6 + 6O2 —> 6CO2 + 6H2O + Energy (ATP)
General principles of cellular catabolism including types of catabolic processes.
Metabolic pathways that release stored energy by breaking down complex molecules. (EX: Fermentation, Aerobic Respiration)
Oxidation reactions.
The complete or partial loss of electrons from a substance involved in a redox reaction.
Reduction Reactions
The complete or partial addition of electrons to a substance involved in a redox reaction.
O.I.L.R.I.G
Oxidation is Los Reduction is Gain (of Electrons)
How do electron carries work?
Electron carriers are small organic molecules involved in the transfer or shuttling of electrons from one molecule to another. When these molecules lose electrons, they become oxidized, whereas gaining electrons converts them into their reduced forms.
Stages of cellular respiration W/ number of ATP produced.
1. Glycolysis | Breaks down glucose into pyruvate in the cytoplasm, doesn't require oxygen (anaerobic). | 2 ATP (net) |
2. Krebs Cycle | Oxidizes pyruvate to produce energy carriers (NADH and FADH2) in the mitochondria. | 2 ATP |
3. Oxidative Phosphorylation | Uses the energy carriers from the Krebs cycle to generate a large amount of ATP through the electron transport chain and chemiosmosis in the mitochondria. | Around 32 ATP |
Glycolysis - Inputs and Outputs, NADH productions, ATP use and production, Location in the Cell
Inputs and Outputs:
Input: 1 molecule of glucose (C6H12O6) and 2 ATP molecules.
Output: 2 molecules of pyruvate (C3H4O3), 4 ATP molecules (net gain of 2), and 2 NADH molecules.
ATP Production and Consumption:
ATP Consumption: Glycolysis initially requires 2 ATP molecules to be invested.
ATP Production: The process generates a total of 4 ATP molecules.
Net ATP Gain: Therefore, there's a net gain of 2 ATP molecules per glucose molecule.
NADH Production:
Glycolysis produces 2 molecules of NADH.
These NADH molecules carry high-energy electrons that will be used in later stages of cellular respiration (e.g., the electron transport chain) to generate more ATP.
Citric Acid Cycle - Inputs and Outputs, ATP production, NADH and FADH2 production, decarboxylation (The intake of carbon dioxide), Location in the cell.
Inputs and Outputs:
Inputs: Acetyl-CoA, water, NAD+, FAD, and ADP + Pi.
Outputs: CO2, NADH, FADH2, ATP (or GTP), and CoA.
ATP Production:
The cycle directly produces a small amount of ATP (or GTP) through substrate-level phosphorylation in each turn.
The NADH and FADH2 produced in the cycle are then used in the electron transport chain to generate a much larger amount of ATP through oxidative phosphorylation.
NADH and FADH2 Production:
Each turn of the cycle forms three NADH molecules and one FADH2 molecule.
These reduced coenzymes (NADH and FADH2) carry high-energy electrons that are used to generate ATP in the electron transport chain.
Decarboxylation:
Decarboxylation, or the removal of carbon dioxide, occurs in several steps of the cycle.
This process releases carbon dioxide as a waste product.
Location in the Cell:
The citric acid cycle takes place in the matrix of the mitochondria.
The enzymes involved in the cycle are mostly soluble, except for succinate dehydrogenase, which is embedded in the inner mitochondrial membrane.
Electron Transport Chain - Inputs and Outputs, ATP Production, NADH and FADH2 drop-off of electrons, Role of Oxygen, Location in the cell
Inputs and Outputs:
Inputs:
NADH and FADH2: These are reduced electron carriers produced during glycolysis and the citric acid cycle, carrying electrons and protons.
ADP: Adenosine diphosphate, which will be converted to ATP during ATP production.
Oxygen (O2): The final electron acceptor in the chain.
Outputs:
NAD+ and FAD: The oxidized forms of NADH and FADH2, which can then be used again in glycolysis and the citric acid cycle.
ATP: The energy currency of the cell, produced through oxidative phosphorylation.
Water (H2O): Formed when oxygen accepts electrons and protons at the end of the chain.
ATP Production:
The ETC consists of a series of protein complexes embedded in the inner mitochondrial membrane.
As electrons move through these complexes, protons (H+) are pumped from the mitochondrial matrix to the intermembrane space, creating a proton gradient.
This proton gradient drives the synthesis of ATP by ATP synthase, an enzyme located in the inner mitochondrial membrane.
This process is called oxidative phosphorylation, where the energy from the electron transport chain is used to generate ATP.
NADH and FADH2 Electron Drop-off:
NADH: Donates its electrons to the first protein complex (Complex I) in the ETC.
FADH2: Donates its electrons to the second protein complex (Complex II) in the ETC.
The electrons then move through the remaining complexes (III and IV) in the chain, eventually being transferred to oxygen.
Role of Oxygen:
Oxygen acts as the final electron acceptor in the ETC.
It combines with electrons and protons (H+) to form water (H2O).
Without oxygen, the electron transport chain would halt, and ATP production would cease.
Location in the Cell:
The electron transport chain is located on the inner mitochondrial membrane.
The inner membrane is folded into cristae, which increases the surface area for the ETC.
This location allows for the efficient transfer of electrons and protons, and the synthesis of ATP.
Chemiosmosis - How is works + Where it works
How it Works:
Proton Gradient:
The electron transport chain (ETC) pumps protons (H+) across a membrane, creating a higher concentration of protons on one side than the other, forming a proton gradient.
ATP Synthase:
A protein complex called ATP synthase acts like a turbine, allowing protons to flow down their concentration gradient across the membrane.
ATP Synthesis:
As protons flow through ATP synthase, the enzyme uses the energy released to convert ADP (adenosine diphosphate) and inorganic phosphate into ATP (adenosine triphosphate), the cell's energy currency.
Where it Works:
Mitochondria:
In cellular respiration, chemiosmosis occurs in the inner mitochondrial membrane, where the ETC is located, and ATP synthase is embedded.
Chloroplasts:
In photosynthesis, chemiosmosis occurs across the thylakoid membrane, where the ETC is located, and ATP synthase is embedded.
Bacteria and Archaea:
Many bacteria and archaea also utilize chemiosmosis for ATP production, often across their plasma membrane
ATP Synthase
ATP synthase is a crucial enzyme that acts as a molecular motor, using the energy from a proton gradient to synthesize ATP (adenosine triphosphate) from ADP (adenosine diphosphate) and inorganic phosphate, powering cellular processes.
Total energy Yields of Glucose Cellular Respiration
Around 32 ATP
Aerobic Respiration Vs. Anaerobic Respiration
Aerobic respiration requires oxygen and produces significantly more ATP (energy) than anaerobic respiration, which occurs without oxygen and is less efficient. (2 ATP Made through this process.)
Fermentation
Goes through Glycolysis but doesn’t have oxygen to go through cellular respiration.
Lactic acid fermentation: Used to make sauerkraut, kimchi, pickles, yogurt, and sourdough bread
Ethyl alcohol fermentation: Used to make wine and beer
Acetic acid fermentation: Used to make vinegar and condiments
Versatility of catabolism and explain how fats and proteins are metabolized.
Fats are metabolized through beta-oxidation, while proteins are broken down into amino acids, which can then be used for energy or other metabolic processes.
Regulation of Cellular Respiration by feedback.
Cellular respiration is regulated through feedback mechanisms, where the end products or intermediates of the pathways influence the activity of key enzymes, ensuring a balanced energy supply and preventing overproduction of ATP.