28629 L6 - genetic variability in phase I and II drug metabolising enzymes
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
intro to pharmacogenetics:
Long known that there are differences in drug metabolism – between species, between strains of animals, between individuals
Pharmacogenetics is the study of the genetic basis of variations in drug metabolism
Polymorphisms are changes in DNA sequence in drug metabolising genes
Different “forms” of the same gene are called alleles
Allelic variation is common in human populations
Important factor in inter-individual differences in drug metabolism along with environmental factors like diet – enzyme inducers, enzyme inhibitors
Variation affects pharmacokinetics of drugs
Effects of polymorphisms on drug metabolism:
No effect
Reduce efficacy
Increase activity – associated with toxicity
CYP2D6 polymorphisms:
Around 1000 polymorphisms with multiple outcomes - CYP 2D6*4 most prevalent (decreased)
normal
increased
decreased
no activity
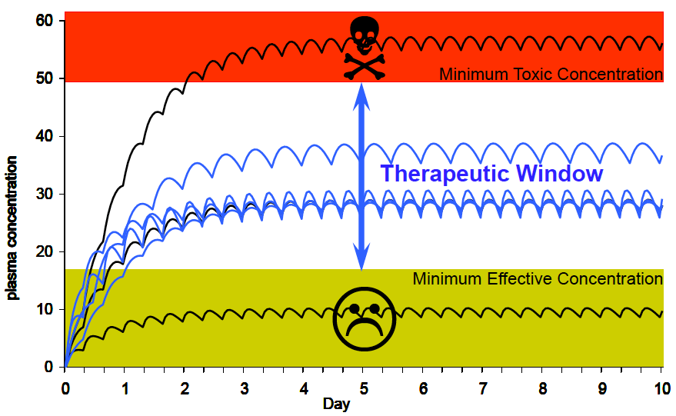
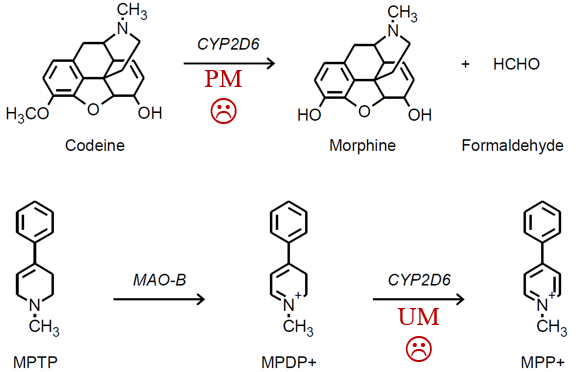
Poor Metabolizers (PM) (little or no function):
slower drug metabolism
higher peak plasma levels (3–4×)
higher area under the curve (AUC, >4×)
higher risk of adverse drug reaction
no response from certain prodrugs e.g. codeine
Ultra-rapid Metabolizers (UM) (multiple 2D6 copies)
Lower peak plasma levels and AUC
Little drug response at ordinary dosage
Examples of drugs metabolised by CYP2D6:
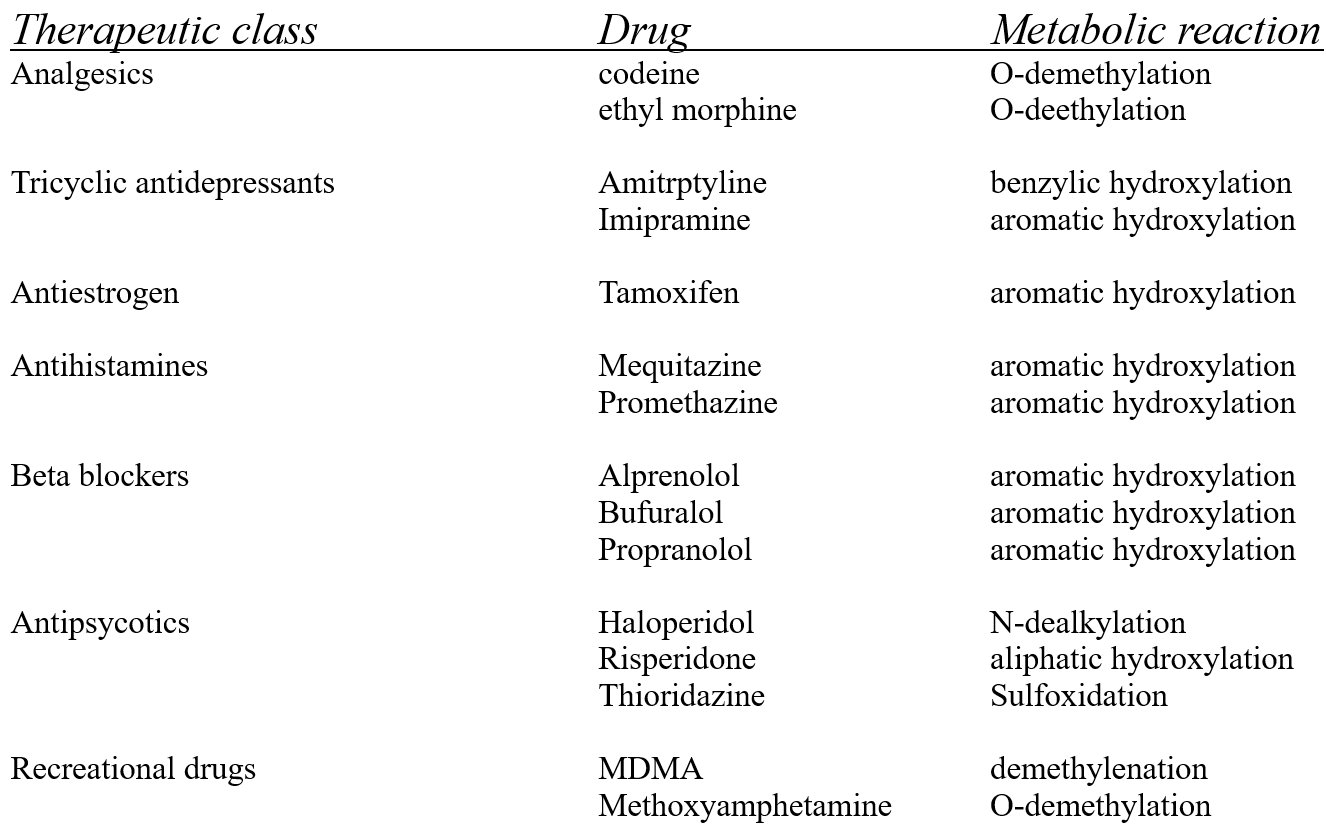
CYP 2D6 different drugs different outcomes:
rapid metabolisers - produce more of the toxic product
poor metabolisers - produce less of the active product
maybe check this in the recording
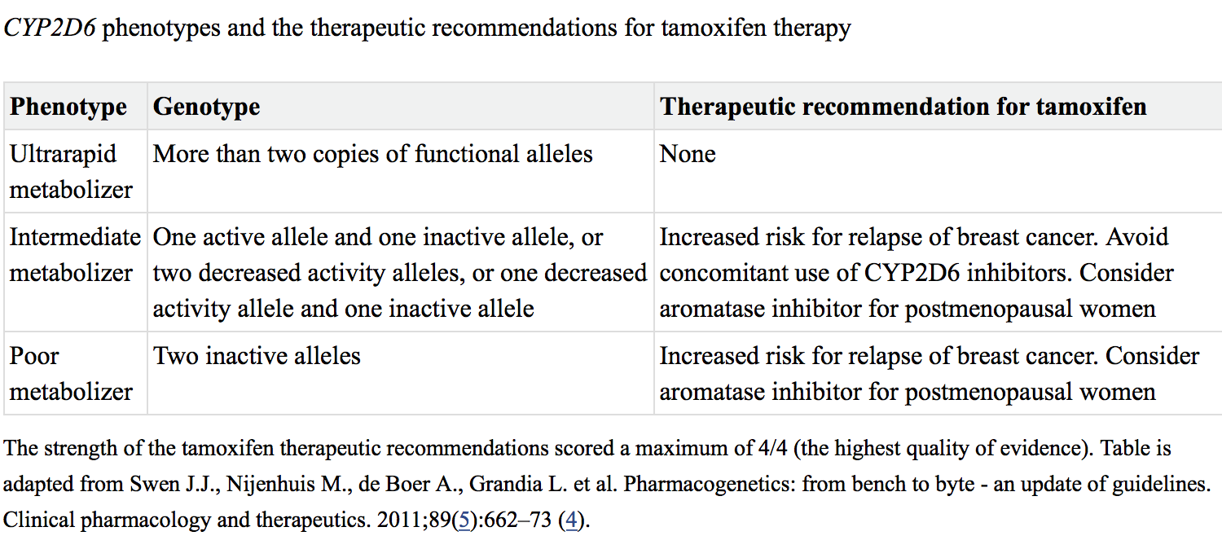
Tamoxifen metabolism and CYP2D6:
Tamoxifen is the major adjuvant therapy for ER-positive breast cancers in premenopausal woman
Metabolism of TAM to the active metabolites 4OH-TAM and Endoxifen mainly undertaken by CYP2D6
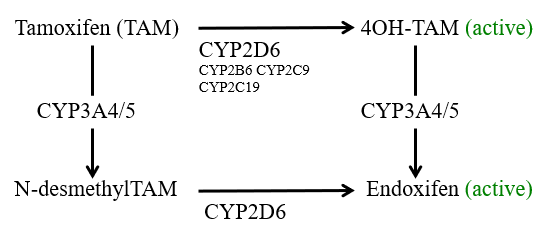
Does CYP2D6 genotype affect clinical outcome in women taking tamoxifen?
FDA-approved drug label does not discuss genetic testing for CYP2D6
National Comprehensive Cancer Network does not recommend CYP2D6 testing as a tool to determine endocrine treatment
Dutch Pharmacogenetics Working Group has made recommendations for tamoxifen therapy based on CYP2D6 genotypes
CYP2C9 and warfarin:
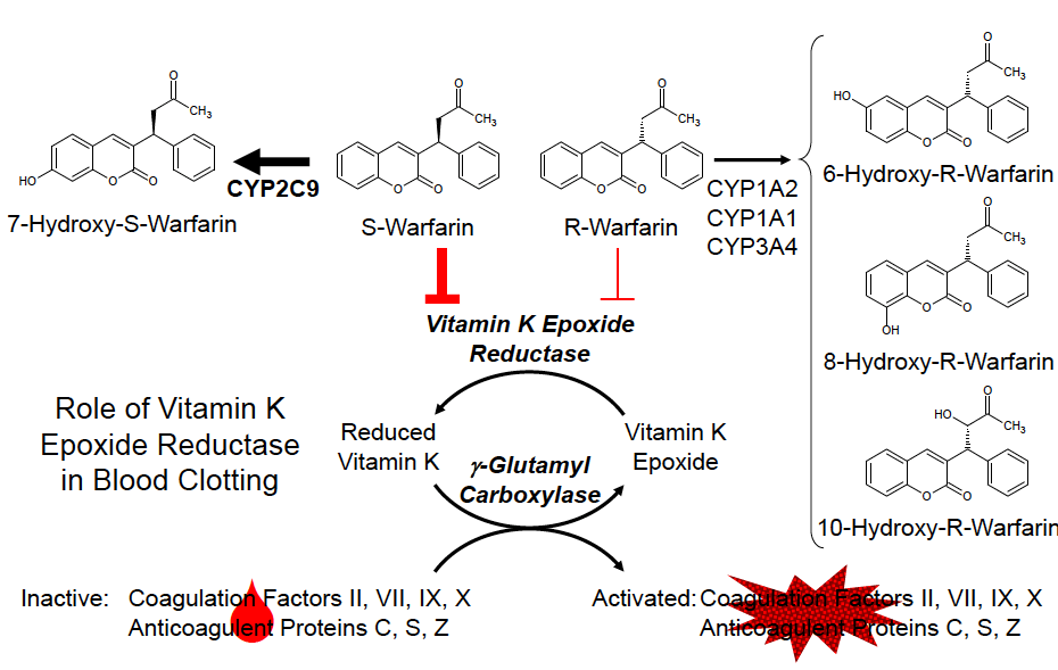
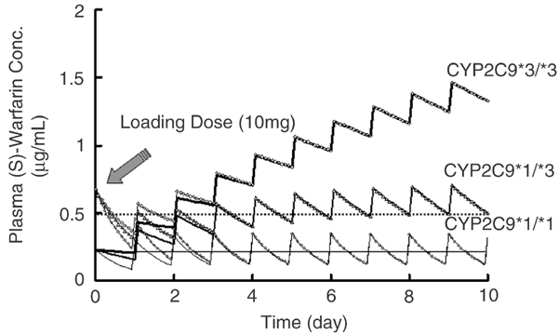
CYP2C9 is polymorphic in the human population:
Around 800 known polymorphisms
CYP2C19*2, 3 and 4 all have reduced activity towards S-Warfarin
Increased abnormal bleeding in patients with variant CYP2C9 alleles (*2 - 4*)
The FDA-approved drug label for warfarin states: “that CYP2C9 and VKORC1 genotype information, when available, can assist in the selection of the initial dose of warfarin”
phase II polymorphisms:
noted in majority of phase II enzymes
tend to be of lesser clinical/toxicological significance
e.g.
N-acetylation
glucuronidation
sulfonation
glutathione conjugation
polymorphisms can occur in both the enzyme + in enzymes involved in production of a cofactor e.g. PAPS, UDP-glucuronic acid
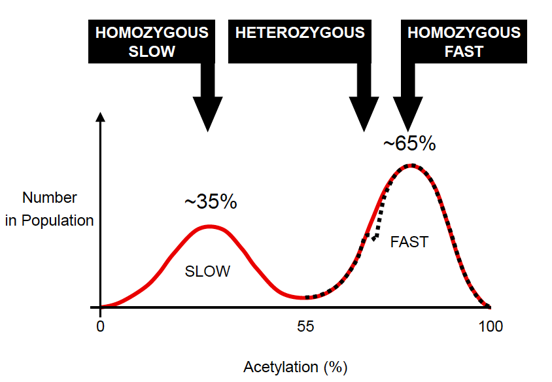
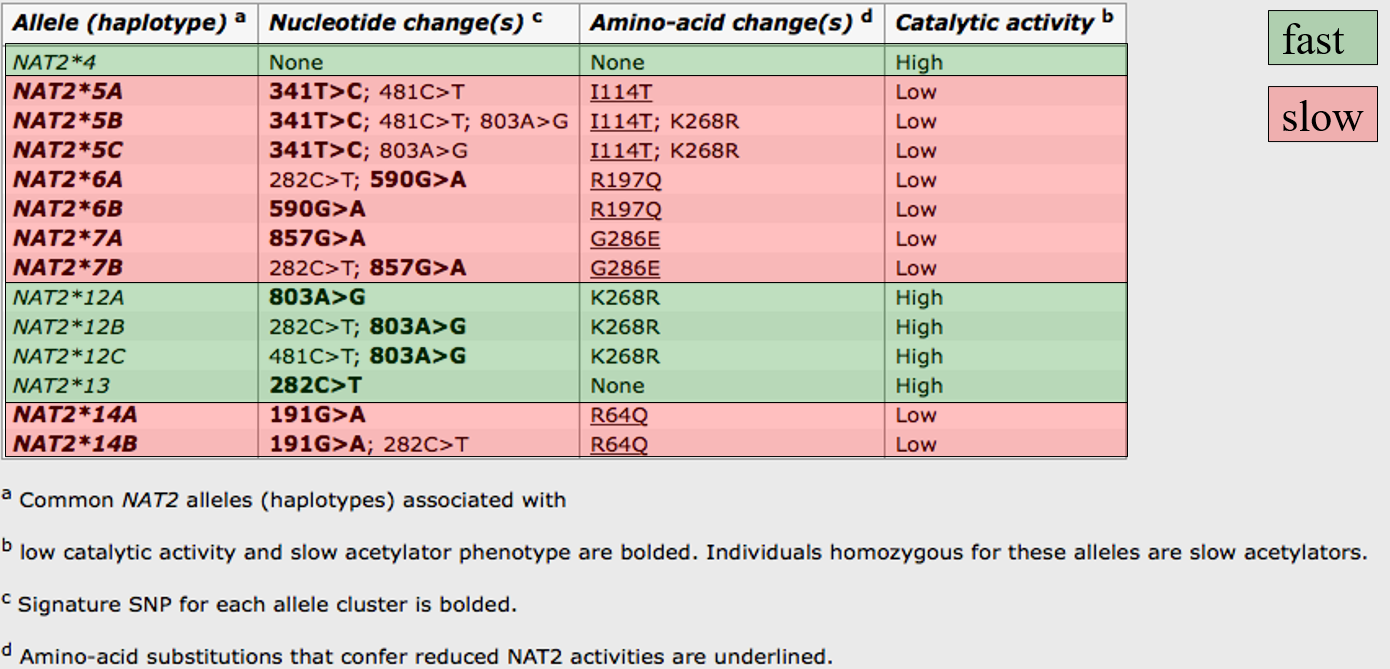
N-acetyl transferases (NATs):
2 known functional isoforms of NAT in humans (NAT1 and NAT2)
Individuals can be phenotyped based on activity towards “probe drugs” e.g. sulfamethazine, isoniazid
About 1/3 of the population have a “slow” acetylator phenotype
Autosomal recessive trait
Many NAT2 alleles exist: at least 60
NAT2 role in toxicity of aryl amines:
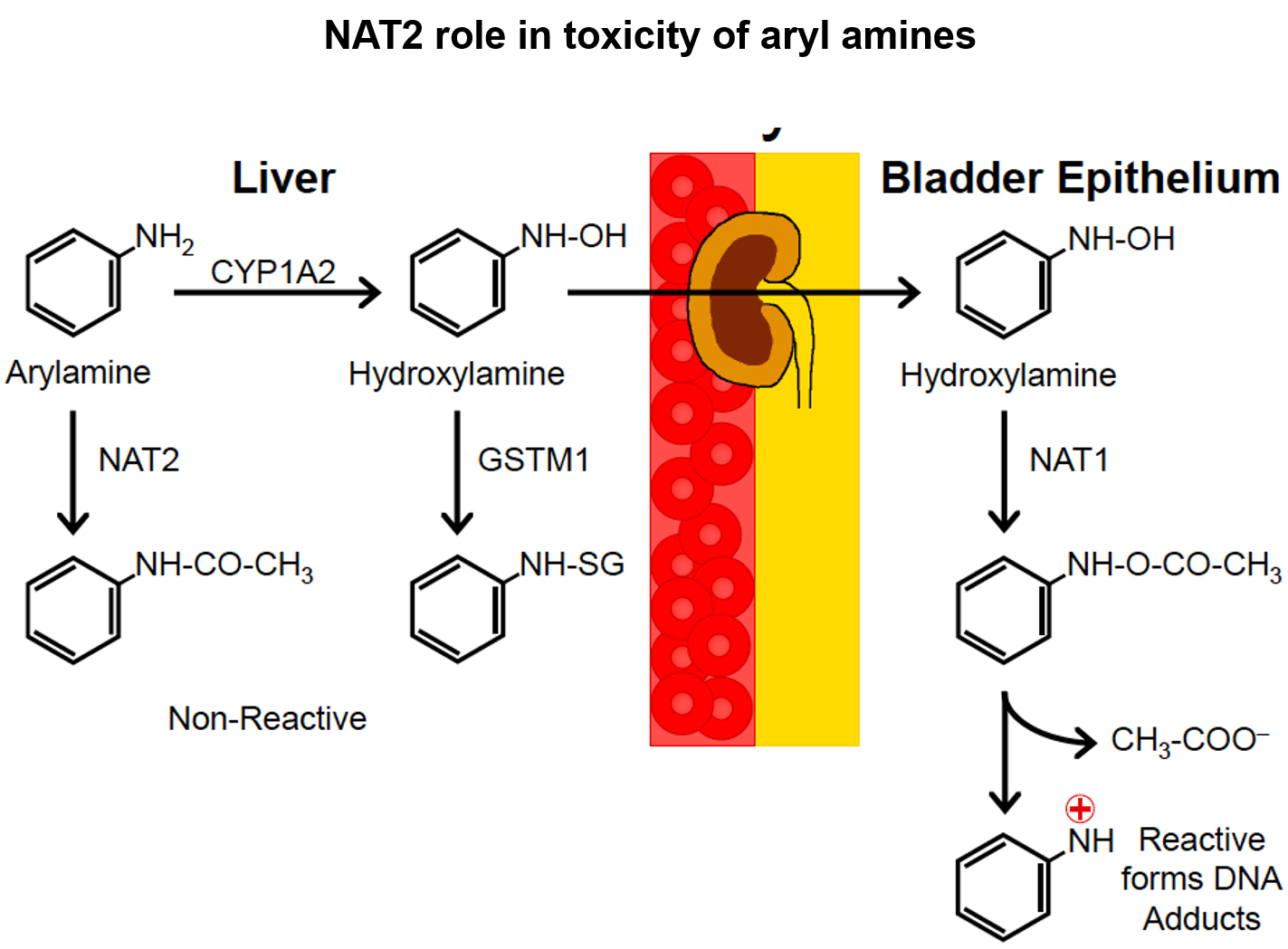
activation of carcinogen arylamines:
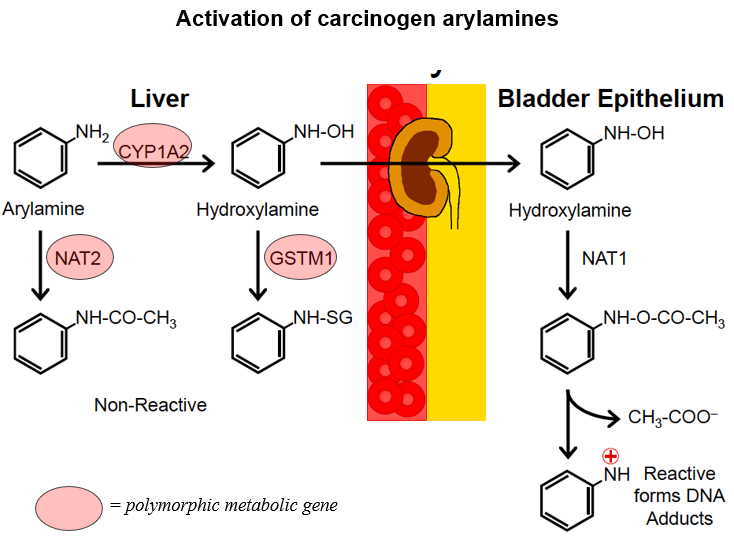
Case study 4: UGT1A1
At least 16 functional isoforms of UGT in 2 subfamilies:
UGT1 family – 10 isoforms
UGT2 family – 6 isoforms
Numerous polymorphisms have been identified including more than 100 variants of UGT1A1
UGT1A1*28 and sensitivity to ironotecan
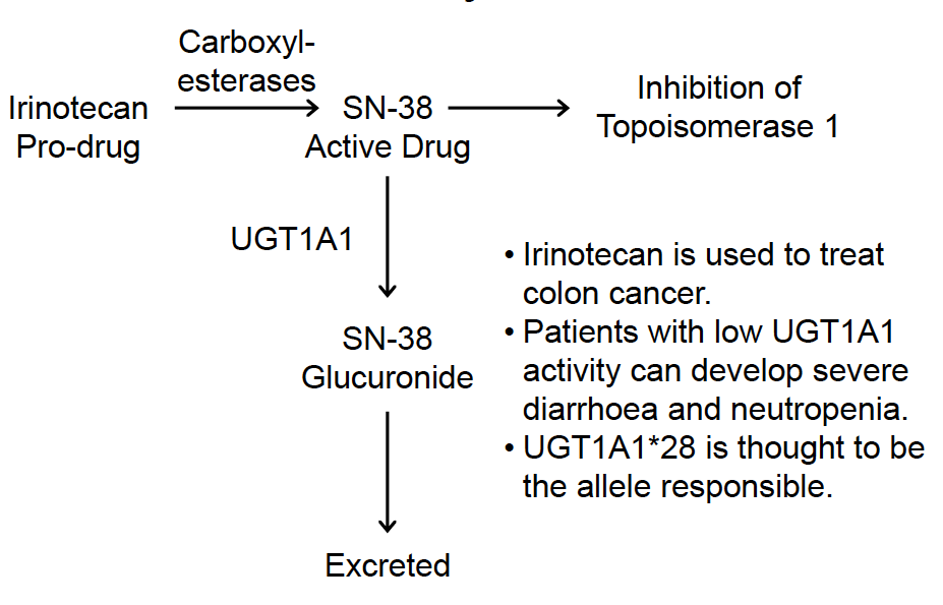
Effect of UGT1A1*28 on pharmacokinetics of ironotecan and incidence of neutropenia:
The FDA-approved drug label for irinotecan states that “when administered as a single-agent, a reduction in the starting dose by at least one level of irinotecan hydrochloride injection should be considered for patients known to be homozygous for the UGT1A1*28 allele”
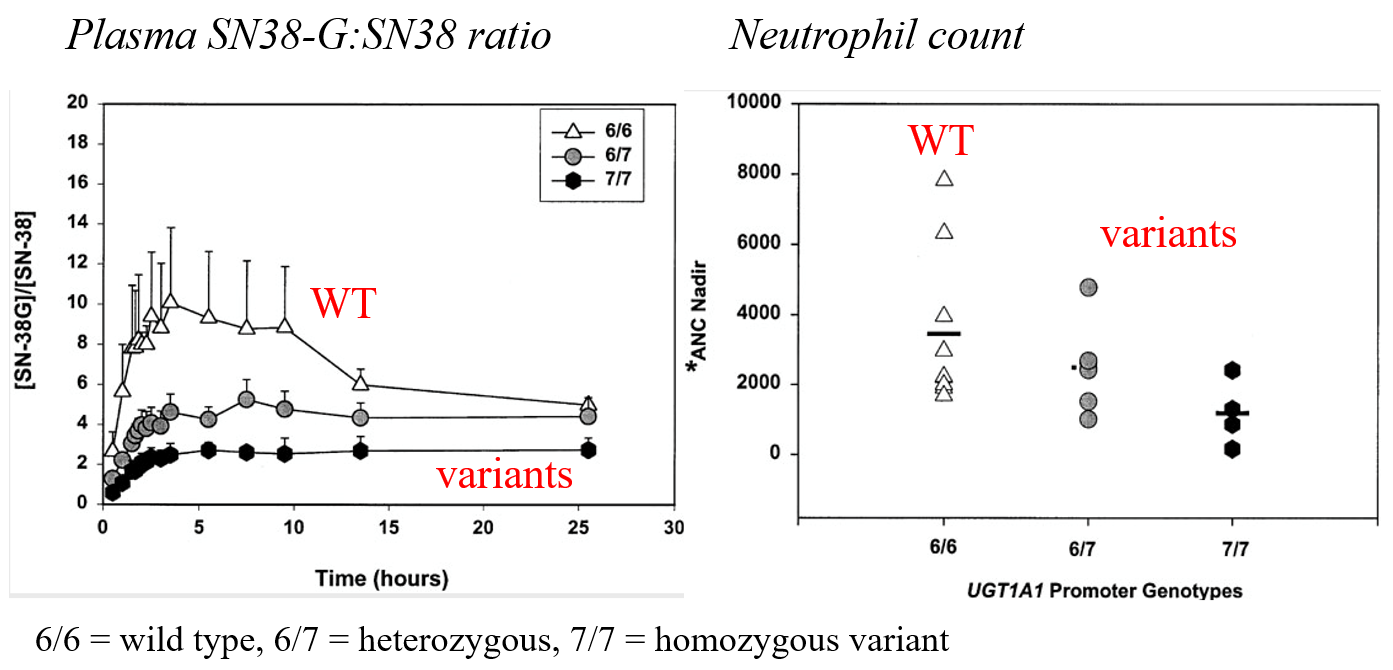
Case study 5: GSTs
Major important polymorphisms are in GSTM family of genes
GSTM1, GSTM3 and GSTM4 are known to be polymorphic.
GSTM1*0 is a common deletion variant:
Ethnic Group Frequency
Pacific Islander/Malay 62 – 100%
European 35 – 62%
Asian 32 – 53%
Hispanic 40 – 53%
African 23 – 41%
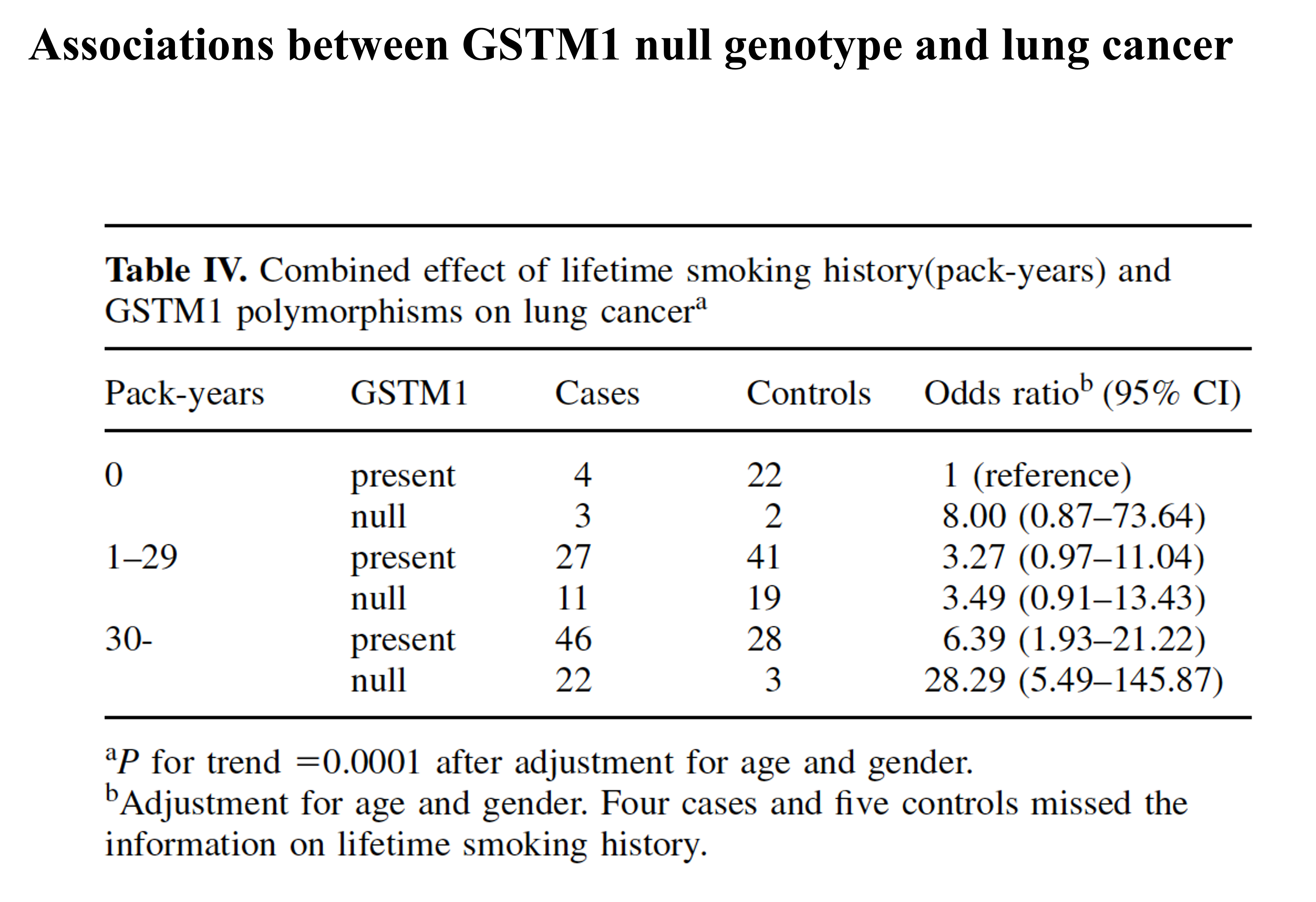
GSTM1*0: increased risk of asthma, sensitivity to some chemicals?
Associations between GSTM1 null genotype and lung cancer:
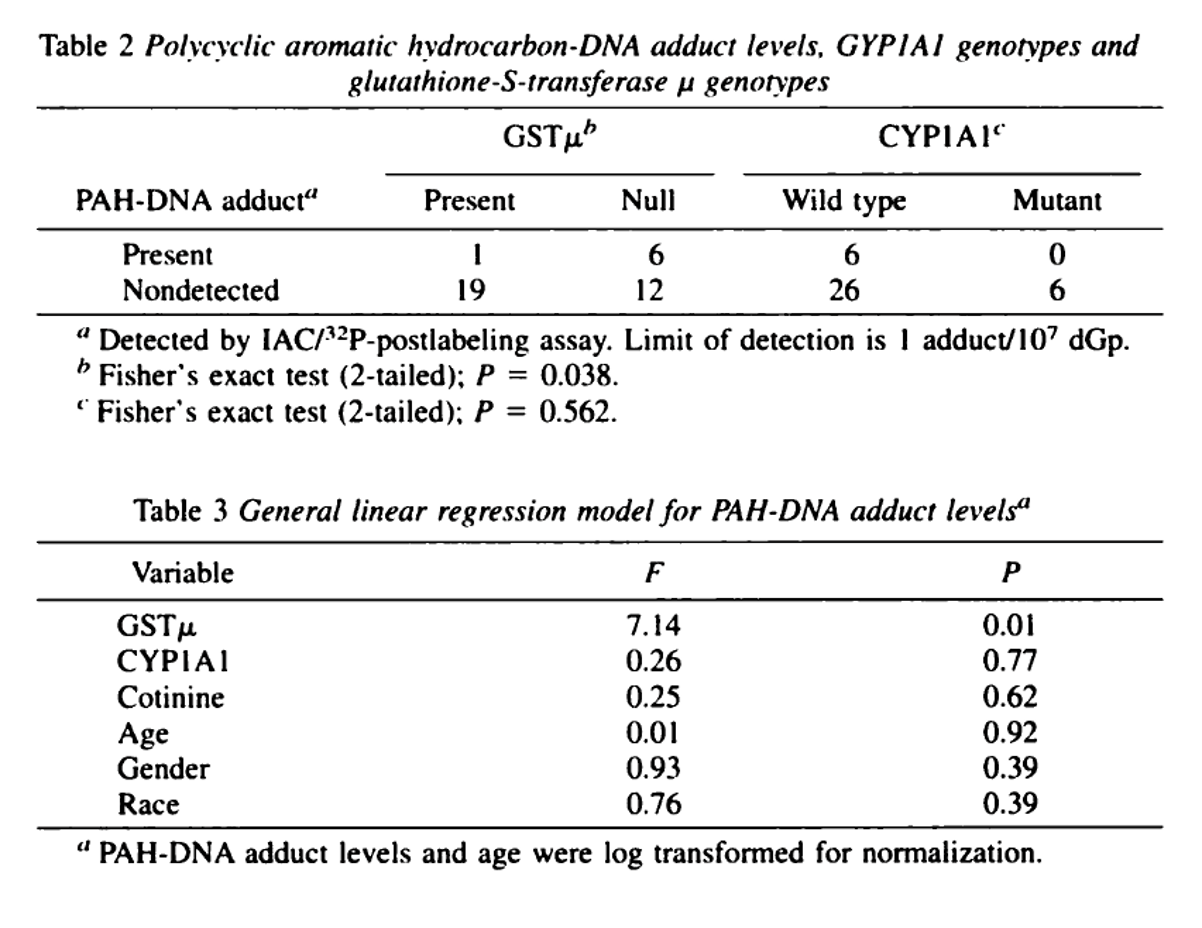
Interaction between SNPs in CYP1A1 and GSTM1 null genotype and levels of BaP adducts:
conclusions:
Almost all drug metabolism and drug transporter genes are polymorphic
These polymorphisms can greatly influence drug metabolism
Partly responsible for inter-individual variation in pharmacokinetics and drug toxicity
None of these polymorphism exist in isolation
Multiple polymorphisms are very likely to interact – need to consider the whole pathway
Also need to consider environmental factors e.g. diet